Abstract
Four phosphonate derivates of 2,3-diphosphoglycerate (2,3-DPG), in which the phosphate group is replaced by a methylene or difluoromethylene, were successfully synthesized for use as allosteric modulators of hemoglobin (Hb) O2 affinity. The syntheses were accomplished in four steps and the reagents were converted to their potassium salts to allow for effective binding with Hb in aqueous media. O2 equilibrium measurements of the chemically modified Hbs exhibited P50 values in the range 8.9–12.8 with Hill coefficients in the range of 1.5–2.4.
Introduction
Development of a suitable red blood cell (RBC) substitute, which can be stored at ambient temperature and be infused safely regardless of recipient blood type, has been of significant interest to the scientific community. Efforts to find a safe and effective replacement for RBCs date back to the 17th century (Winslow Citation1995, Palmer et al. Citation2009). In recent years, the use of artificial O2 carriers has received significant attention due to public concerns over the safety of blood products, complications from blood transfusion, and postpartum treatment of hemorrhage. Progress in the field was accelerated by the emergence of the human immunodeficiency virus (HIV) which is epidemic in the 1980s and the realization that infectivity was transmissible via blood, since the use of blood replacement fluids avoids many of the adverse consequences of blood transfusion (Schreiber et al. Citation1996).
Perfluorocarbons were the first class of materials to advance to human trials and receive FDA approval, while an alternate approach was based on cell-free hemoglobin (Hb). The use of cell-free Hb as a blood substitute is associated with two major problems. First, it suffers from a very short circulation lifetime in the blood stream, mainly due to its breakdown from a large tetrameric protein, α2β2, into two smaller αβ dimeric units, consequently facilitating its rapid renal elimination and afflicting considerable renal toxicity. Second, cell-free Hb exhibits a P50 value of 12–14 mm Hg (partial pressure of O2 at which half of the O2 binding sites on the Hb molecule are saturated). This is significantly lower than that of whole blood which exhibits a P50 of 27 mm Hg. With such a strong affinity for O2, Hb's ability to release oxygen to surrounding tissues is greatly reduced thus limiting its ability to act as an effective blood substitute (Bakker et al. Citation1992, Gould et al. Citation1992, Spahn Citation2000). The drawbacks of stroma-free Hb have been attributed to the loss of the natural allosteric effector, 2,3-disphosphoglycerate (2,3-DPG), upon its isolation from the red blood cell (Horton Citation1993, Astrup and Rorth Citation1971). This work seeks to develop novel analogs of 2,3-DPG that will maintain the integrity of the tetrameric protein and mimic the P50 of whole blood.
Recently, phosphonates have been identified as important mimics of phosphates. This is particularly so in the field of phosphate chemistry, owing to the great importance of phosphate-containing molecules in biological processes (Thatcher and Campbell Citation1993). Unlike a phosphate group, the phosphonate linkage is not readily hydrolyzed in aqueous solution, and this unique property has made these compounds attractive as phosphate analogs in numerous applications (Engel Citation1977, Tozer and Herpin Citation1996, Blackburn Citation1981). The structural correspondence of C–C–P bonds with C–O–P bonds, although significantly dissimilar in their chemical characteristics, has provided an avenue to systematic chemical and biomedical studies of phosphonic acids and their derivatives (Wiemer Citation1997, Waschbüsch et al. Citation1997, Moonen et al. Citation2004, Palacios et al. Citation2005, Caplan et al. Citation1998, Stremler and Poulter Citation1987, Chambers et al. Citation1990). The driving force for the development of analogs of the phosphate moiety is the unwanted lability of the ester P–O bond. There are numerous examples of enzyme inhibitors in the literature incorporating phosphonates as phosphate mimics (Burke et al. Citation1993). Furthermore, phosphonates have been used successfully to mimic monophosphate and triphosphate nucleotides (Blackburn and Kent Citation1981).
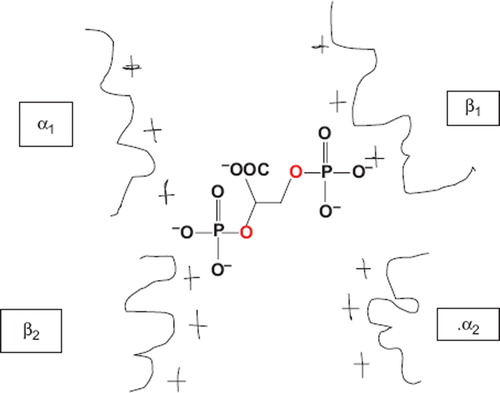
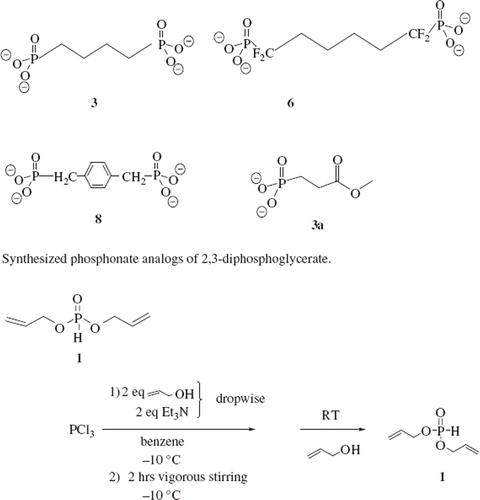
In this study, a series of analogs of 2,3-DPG () containing phosphate isosters were synthesized and characterized for use as site-directed affinity reagents for human Hb.
Methods: experimental procedure
Materials and methods
Reagents and chemicals were purchased from Fischer Scientific and Sigma-Aldrich. Commercial reagents and chemicals were used without further purification. Solvents were dried over sodium metal prior to use. Reagents used for preparation of buffers were analytical grade. Human Hb was obtained from human RBCs via tangential flow filtration (a). 1H, 13C and 31P NMR spectra were obtained on a Bruker Avance 400 MHz Ultra ShieldTM. Chemical shifts for 1H (400 MHz) and 13C (100 MHz) were referenced to CDCl3 or D2O and reported in ppm. FT-IR spectra were collected using a Nicolet Avatar-IR 370 or a Perkin Elmer series 100 spectrometer. Mass spectra were obtained using an Agilent Technologies 5973 inert mass selective detector. MALDI-TOF analyses were carried out using a Perkin Elmer Voyager-DE STR Biospectrometry workstation equipped with a two-stage acceleration source. Reaction progress was monitored using thin-layer chromatography (TLC) using Silica Gel 60 F254 glass-coated plates. Products were purified with column chromatography using 63–200 μm mesh silica gel or fractional distillation under reduced pressure or recrystallization with acetonitrile.
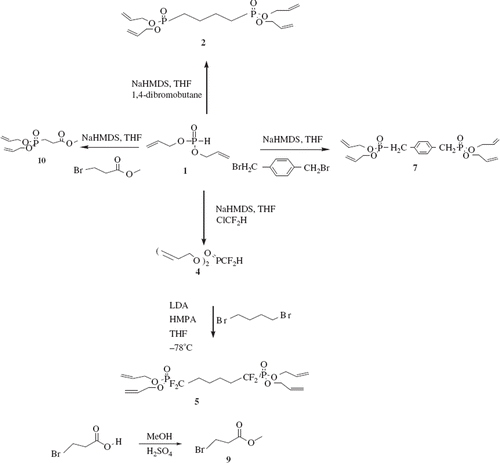
Diallyl phosphonate (1)
In a 500 mL two-neck round bottom flask fitted with an addition funnel, 17.45 mL (200 mmol) of phosphorus trichloride was dissolved in 150 mL of dry benzene at − 10°C. A mixture of 27.27 mL (400 mmol) of allyl alcohol and 55.64 mL (400 mmol) of triethyl amine was added slowly over a period of 1 hrs under vigorous stirring. After allowing the resulting mixture to warm to room temperature, additional 14.13 mL (208 mmol) of allyl alcohol was added. Then the reaction was allowed to stir overnight at room temperature, the triethylamine hydrochloride precipitate was removed by filtration, and washed with benzene, with the washings being added to the liquid reaction product. The organic layer was washed with 100 mL of water, and then dried over anhydrous sodium sulfate. Benzene and other low-boiling impurities were removed under reduced pressure at a temperature less than 50°C. The diallyl phosphonate produced was recovered by reduced-pressure distillation at 68°C–72°C in 87% yield. Spectroscopic analysis: 1H-NMR (400 MHz, CDCl3, δ): 4.5–4.7 (m, 4H) [CH2OP], 5.28–5.39 (dd, 2H) [CH2 = ], 5.41–5.59 (dd, 2H) [CH2 = ], 5.91–6.12 (m, 2H) [ = CH], 7.8 (d, 1H) [PH]; 13C NMR (400 MHz, CDCl3, δ) 63.3 [CH2O], 119.1 [CH2 = ], 130.1 [ = CH]; MS (m/z, %): 121 (M+-41(CH2 = CH-CH2)), 105 (M+-57(CH2 = CH-CH2O)), 79 (18), 80 (17), 57 (M+-105 (2(CH2 = CH-CH2O))), 41(M+-121(HPO3C3H5)).
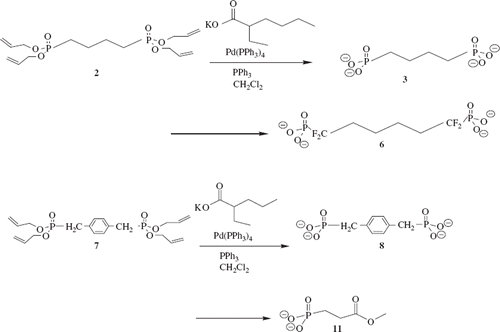
Tetraallyl 1,4-bisphosphonate butane (2)
Sodium hexamethyl disilazane (10.35 mL 2M in THF, 20 mmol) was added to a stirred solution of diallyl phosphonate (1), (3 g in 5 mL THF, 19.0 mmol) at room temperature. The reaction mixture was stirred for 2 hrs before dropwise addition of 1,4-dibromobutane (0.9 mL 7.5 mmol). The reaction was stirred overnight at room temperature affording the sodium bromide precipitate which was removed by filtration through Celite and washed with THF. The washings were added to the liquid reaction product. The solvent was evaporated at reduced pressure and purified via column chromatography (50/50,ethyl acetate:hexane) to afford brown oil with 78%. Spectroscopic Analysis:1H-NMR (400 MHz, CDCl3, δ):1.8–1.9 (dd, 4H) [CH2CH2P], 1.95–2.1 (m, 4H) [CH2P], 4.5–4.7 (m, 8H) [CH2OP], 5.28–5.39 (dd, 4H) [CH2 = ], 5.41–5.59 (dd, 4H) [CH2 = ], 5.91–6.12 (m, 4H) [ = CH]; 13C NMR (400 MHz, CDCl3, δ) 32.1 [CH2CHP], 35.1 [CH2P], 65.3 [CH2O], 119.1 [CH2 = ], 133.1 [ = CH]; MALDI M + 1 379.7379.
Tetrapotassium 1,4-bisphosphonate butane (3)
To Compound 2 (1.5 g, 4.0 mmol) in 10 mL dichloromethane, potassium 2-ethylhexanoate (4.37 g, 24.0 mmol), tetrakis(triphenylphosphine)palladium(0) (0.36 g, 0.32 mmol), and triphenylphosphine (0.36 g, 1.52 mmol) were added and the mixture was stirred at 20–25°C in the dark until all starting materials had reacted as determined by TLC. The reaction was then allowed to stir overnight at room temperature in the dark. The mixture was then diluted with 20 mL of ethyl acetate, and the solvent was evaporated. The residue was washed with ether, and dried in vacuo to afford the crude product. Trituration with ethyl acetate/methanol (95/5) gave the pure 3 in 42% yield as a water soluble hygroscopic white powder. Spectroscopic Analysis: 1H NMR (400 MHz, CDCl3, δ): 1.2–1.3 (d, 2H) [CH2CH2P], 1.3–1.4 (d, 2H) [CH2P]; 31P NMR (100 MHz, PPh3, δ): 20; ESI-MS: m/z 371.1017 ([M + H]+ calcd 369.8344).
Diallyl (α,α-Difluoromethyl)phosphonate (4)
To a solution of diallyl phosphonate (1) (7 g, 43.21 mmol) in 10 mL THF at room temperature was added sodium bis(trimethyl)amide (23.9 mL of a 2 M solution in heptanes/ethylbenzene). The reaction mixture was allowed to stir for 40 min. Chlorodifluoromethane (Freon 22) was bubbled into the solution for 40 min, and the reaction mixture was stirred for additional 12 hrs under balloon atmosphere of chlorodifluoromethane. Precipitated NaCl was removed by filtration through Celite and the solvent was evaporated. The resulting liquid was distilled to yield 4 as a pale yellow liquid Spectroscopic analysis; 1H NMR (400 MHz, CDCl3, δ): 4.71 (m, 4H) [CH2O], 5.28–5.39 (dd, 2H) [CH2 = ], 5.41–5.89 (br dd, 2H) [CH2 = ], 5.90 (dd, 1H) [CF2H], 5.91–6.12 (m, 2H) [ = CH]; 13C NMR (400 MHz, CDCl3, δ) 68.3 [CH2O], 69.9 [CF2], 119.9 [CH2 = ], 140.1 [ = CH]; MALDI: M + 1 216.1101.
Tetraallyl 1,1,6,6-tetrafluorohexane-1, 6-bisphosphonate (5)
A solution of 4 (2 g, 9.4 mmol) in THF (10 mL) was cooled in a dry ice-acetone bath, and treated via syringe with a solution of lithium diisopropylamide (4.7 mL, 2 M in THF/ n-heptane/ethylbenzene) and 1.63 mL hexamethylphosphoramide. After stirring for 2 hrs, solution was cannulated into a cold solution (− 78°C) of 1,4-dibromobutane (2 g, 9.4 mmol) in THF (10 ml). The reaction mixture was allowed to warm to room temperature, concentrated, and quenched with 0.5 N HCl, and extracted with EtOAc (2 × 100 mL). The organic layer was washed with 1 N HCl (4 × 10 mL), saturated NaHCO3 (20 mL), and saturated NaCl (20 mL). The EtOAc solution was dried over Na2SO4, filtered, and evaporated. The oily residue was chromatographed on silica gel (90% CH2Cl2:10% MeOH as eluent) to give 5 as a yellow oil in 81% yield. Spectroscopic Analysis: 1H-NMR (400 MHz, CDCl3, δ): 1.36–1.48 (m, 4H) [CH2CH2CF2], 1.78–2.00 (m, 4H) [CH2CF2], 4.50–4.71 (m, 4H) [CH2O], 5.28–5.41 (dd, 2H) [CH2 = ], 5.42–5.60 (br dd, 2H) [CH2 = ], 5.93–6.13 (m, 1H) [ = CH]; 13C-NMR (400 MHz, CDCl3, δ) 32.10 [CH2], 35.10 [CH2CF2], 68.3 [CH2O], 71.1(d) [CF2], 119.0 [CH2 = ], 133.1 [ = CH].
Tetrapotassium 1,1,6,6-tetrafluorohexane-1, 6-bisphosphonate (6)
To a solution of 5 (3.0 g, 6.45 mmol) in dichloromethane (10 mL) was added potassium 2-ethylhexanoate (6.29 g, 34.0 mmol), tetrakis(triphenylphosphine)palladium(0) (1.0 g, 0.882 mmol), and triphenylphosphine (1.05 g, 3.8 mmol). The mixture was stirred at 20–25°C in the dark until all starting materials had reacted as determined by TLC. The reaction was then allowed to stir overnight at room temperature in the dark. The mixture was diluted with 20 mL of ethyl acetate, and the solvent was evaporated. The residue was washed with ether, and dried in vacuo to afford the crude product. Triturating with ethyl acetate/methanol (95/5) gave 6 in 41% yield as a water soluble hygroscopic white powder. Spectroscopic Analysis: 1H-NMR (400 MHz, D2O,δ): 1.20–1.27 (m, 4H) [CH2CH2CF2], 1.38–1.51 (m, 4H) [CH2CF2]; 31P-NMR (100 MHz, PPh3, δ): 5.5 (t, J = 99.8 Hz); ESI-MS: m/z 470.8910([M + 1] calcd 469.8280).
Tetraallyl α,α-diphosphonate-p-xylene (7)
To a clean dry 250 mL round-bottomed flask was added diallyl phosphonate (1) (4.5 g, 28.5 mmol) and 10 mL dry THF. The resulting mixture was stirred for 10 min. Sodium hexamethyldisilazane (15.6 mL, 30 mmol) in THF was added slowly and the mixture was stirred for an additional 2 hrs at room temperature. To the reaction mixture was added α,α-dibromobenzene (2.97 g, 11.25 mmol), the reaction mixture was stirred at room temperature for 16 hrs. Precipitated NaBr was removed by filtration through Celite, and the solvent was evaporated. The resulting red yellow oil was purified by column chromatography on silicagel and eluted with ethyl acetate/hexane (60% ethyl acetate: 40% hexane) to obtain 7 as orange oil with 80% yield. Spectroscopic Analysis: 1H-NMR (400 MHz, CDCl3, δ): 3.50–3.45 (d, 4H) [CH2P], 4.5–4.7 (m, 8H) [CH2OP], 5.28–5.39 (dd, 4H) [CH2 = ], 5.41–5.59 (dd, 4H) [CH2 = ], 5.91–6.12 (m, 4H) [ = CH], 7.25–7.32 (dd, 4H); 13C-NMR (400 MHz, CDCl3, δ) 31.0 [CH2P], 32.1 [CH2P], 65.3 [CH2O], 119.1 [CH2 = ], 128.0 [CH2 = , Ar], 131.0 [CH2 = , Ar], 133.1 [ = CH],; MS (m/z): (M-1)+ 425.
Tetrapotassium α,α-diphosphonate-p-xylene (8)
To compound 7 (6.0 g, 14.0 mmol) in dichloromethane (20 mL) was added potassium 2-ethylhexanoate (7.28 g, 39.92 mmol), tetrakis(triphenylphosphine)palladium(0) (1.2 g, 1 mmol), and triphenylphosphine (1.2 g, 4.5 mmol). The mixture was stirred at 20–25°C in the dark until all starting materials had reacted as determined by TLC. The reaction was allowed to stir overnight at room temperature in the dark. The mixture was next diluted with 20 mL of ethyl acetate, and the solvent was evaporated. The residue was washed with ether and dried in vacuo to afford the crude product. Trituration with ethyl acetate/methanol (95/5) gave the pure 8 in 38% yield as a water soluble hygroscopic white powder. Spectroscopic Analysis: 1H-NMR (400 MHz, CDCl3,δ): 3.50–3.45 (d, 4H) [CH2P], 7.25–7.32 (dd, 4H); ESI-MS: m/z 422.2236 ([M + 1] calcd 417.8344).
3-bromo methylpropionate (9)
A solution of 3-bromopropionic acid (10 g, 0.065 mmol) in methanol (125 mL) was cooled to 0°C in a dried round bottom flask. Sulfuric acid (5 mL) was added to the cold solution. The reaction mixture was refluxed for 6 hrs and the solvent was evaporated. The residue was dissolved in CH2Cl2 and washed with water, saturated sodium bicarbonate and sodium chloride. The combined organic layers were dried over anhydrous sodium sulfate. The organic solution was concentrated in vacuo to give brown oil. The resulting oil was purified by column chromatography on silica gel (CH2Cl2/hexane; 95:5) to give red oil with 63% yield. Spectroscopic Analysis: 1H-NMR (400 MHz, CDCl3,δ): 2.85–2.91 (t, 2H) [CH2C(O)], 3.5–3.59 (t, 2H) [CH2Br], 3.69–3.71 (s, 3H) [OCH3]; 13C-NMR (400MHz, CDCl3, δ): 27 [CH2C(O)], 38 [CH2Br], 53 [CH3O], 171 [C = O]; IR: 1734 cm− 1[C = O]; 166 [M-1]+, 135 [CH3O]+, 87 [M-Br].
Diallyl 3-phosphonate methylpropionate (10)
To a clean dry 250 mL round-bottomed flask was added diallyl phosphonate (1) (4.0 g, 24.6 mmol) and dry THF (10 mL). The resulting mixture was stirred for 10 min. Sodium hexamethyldisilazane in THF (12.9 mL, 25.8 mmol) was added dropwise and stirring was continued for additional 2 hrs at room temperature. Then 3-bromo methylpropionate (3.25 g, 19.5 mmol) was added slowly and the reaction mixture was stirred at room temperature for 16 hrs. Precipitated NaBr was removed by filtration through Celite, and the solvent was evaporated. The resulting reddish yellow oil was purified by column chromatography on silicagel and eluted with ethyl acetate/hexane (50/50) to obtain 10 as brown red oil with 49% yield. Spectroscopic Analysis: 1H-NMR (400MHz, CDCl3, δ): 2.85–2.91 (t, 2H) [CH2C(O)], 3.5–3.59 (t, 2H) [CH2P], 3.69–3.71 (s, 3H) [OCH3], 4.5–4.7 (m, 8H) [CH2OP], 5.28–5.39 (dd, 4H) [CH2 = ], 5.41–5.59 (dd, 4H) [CH2 = ], 5.91–6.12 (m, 4H) [ = CH]; 13C-NMR (400 MHz, CDCl3, δ) 27 [CH2C(O)], 28 [CH2P], 53 [CH3O], 65.3 [CH2O], 119.1 [CH2 = ], 133.1 [ = CH], 171 [C = O]; MS (m/z): 249 (M + 1)+.
Dipotassium 3-phosphonate methylpropionate (11)
To a solution of the ester 10 (3.0 g, 12.1 mmol) in dichloromethane (10 mL) was added potassium-2-ethylhexanoate (6.29 g, 34.5 mmol), tetrakis(triphenylphosphine)palladium(0) (1.025 g, 0.882 mmol), and triphenylphosphine (1.05 g, 4 mmol). The mixture was stirred at 20–25°C in the dark until all starting materials had reacted as determined by TLC. The reaction was then allowed to stir overnight at room temperature in the dark. The mixture was then diluted with 20 mL of ethyl acetate, and the solvent was evaporated. The residue was washed with ether, and dried in vacuo to afford the crude product. Trituration with ethyl acetate/methanol (95/5) gave the pure 11 in 48% yield as a water soluble hygroscopic white powder. Spectroscopic Analysis: 1H-NMR (400 MHz, CDCl3, δ): 1.61–1.69 (t, 2H) [CH2C(O)], 2.39–2.42 (t, 2H), 3.60 (s, 3H); 13C-NMR (400 MHz, CDCl3, δ): 23 [CH2P], 24 [CH2C(O)], 50 [CH3O], 174 [C = O]; 31P NMR (100 MHz, PPh3 δ): 21; ESI-MS: m/z 244.9383 ([M + 1] calcd. 243.9305).
O2 Affinity and Cooperativity of Hb
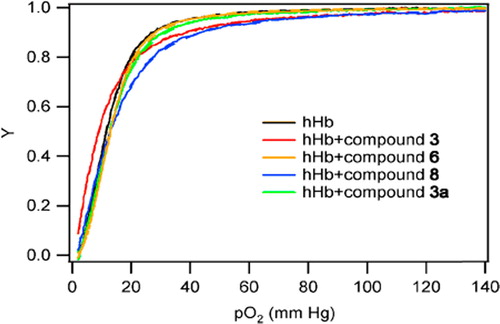
The equilibrium O2 binding curve of Hb exposed to the 2,3-DPG analogs was measured using a Hemox-Analyzer (TCS Scientific Corporation, New Hope, PA) at 37°C. Samples were prepared by thoroughly mixing 50–100 μl of Hb (> 300 mg/mL) with 5 mL of Hemox buffer (pH 7.4), at a 40:1 molar ratio of 2,3-DPG mimic to Hb, 20 μl of Additive-A, 10 μl of Additive-B and 10 μl of anti-foaming agent, all purchased from TCS Scientific Corporation. The samples were allowed to saturate to a pO2 (i.e. partial pressure of O2) of 145 ± 2 mm Hg using compressed air for about 45 mins. After giving the sample enough time to equilibrate, the gas stream was switched to pure N2 to deoxygenate the sample. The absorbance of oxygenated and deoxygenated Hb in solution was recorded as a function of pO2 via dual wavelength spectroscopy. O2-Hb equilibrium curves were fit to a four-parameter (A0, A∞, P50, n) Hill model (Eq. 1), where Y is the fractional saturation of Hb (Y has no units and varies from 0–1, a value of 0 corresponds to fully deoxygenated Hb while a value of 1 corresponds to fully oxygenated Hb), while A0 and A∞ represent the absorbance at 0 mm Hg and full saturation, respectively. P50 represents the pO2 at which the heme-binding sites in the Hb sample are 50% saturated with O2, while n is the Hill coefficient, a measurement of cooperativity in O2 binding.
The goodness of fit was verified by comparing the experimental data ![]() with the curve fitted data
with the curve fitted data ![]() .
.
Results and discussion
Synthesis of Hb allosteric effectors
Initial attempts at synthesizing diallyl phosphonate from allyl alcohol and phosphorous trichloride via Kennedy's method resulted in low yields (Kennedy Citation1957). This prompted us to vary the reaction parameters which included solvent, temperature, base, order of addition, and reaction time. After several experiments, we obtained optimum results by carrying out the reaction at temperatures that ranged from − 10 to − 20°C. Furthermore, we found it necessary to stir the reaction mixture for approximately 2 hrs before addition of the third equivalent of allyl alcohol. The diallyl phosphonate was purified via reduced-pressure distillation and isolated in yields ranging from 85% to 90% ().
Replacement of the P–O bond of 2,3-DPG was achieved by reaction of diallyl phosphonate with chlorodifluoromethane in the presence of sodiumhexamethyldisalazane to afford, α-(diallyl difluoromethyl)phosphonate 4. Subsequent deprotonation of 4 with LDA afforded the lithium salt which was reacted with 1,4-dibromobutane to provide the difluorinated alkyl phosphonate 5. Deprotection of the allyl group gave the tetrapotassium salt 6 which was evaluated as an allosteric effector of Hb. The non-fluroinated phosphonate salts 3 and 8 were also synthesized by reacting two equivalents of diallyl phosphonate with the appropriate dihalide via the Michaelis–Becker reaction () (Berkowitz and Sloss Citation1995). Deprotection of the allyl group gave the desired reagents in good yields.
Synthesis of the dipotassium 3-phosphonate methylpropionate allosteric effector 11 was also prepared from diallyl phosphonate. The diallyl phosphonate was reacted with NaHMDS and methyl 3-bromopropionate in a 1:1 molar ratio. Deprotection gave the dipotassium salt in 90% yield () (Kamber and Just Citation1985).
Evaluation of Hb allosteric effectors
The ideal allosteric effector of Hb is a reagent that closely mimics the chemical structure of 2,3-DPG, while specifically targeting the β-cleft of Hb and causing the least conformational change in the native structure of Hb upon subsequent cross-linking. 2,3-DPG forms a salt bridge with the positively charged amino acid residues on the β globins and controls Hb O2 affinity and stabilizes the tetramer. These novel reagents carry two or more anions, which should guide them towards the β cavity to form a stable tetramer with tunable O2 affinity. Compounds 3, 6 and 8 each consist of four anionic charges and are structurally comparable to DPG. Compound 11 encompasses an ester functional group at one end, in order to make a covalent bond with an amine group on one of the 2 β globin chains.
Structural comparison shows that the four oxygen anions on 3, 6 and 8 are at similar positions to each other as on 2,3-DPG. Although there are only four anionic charges on 3, 6 and 8 compared to 2,3-DPG which has five anionic charges, the anions should direct these phosphonate reagents to the β cleft site to form a salt bridge with the positively charged amino acid residues in this region.
Activities of phophonate analogs of 2,3-DPG on Hb
The effect of phosphonate analogs of 2,3-DPG on the O2 affinity of human Hb was measured using a Hemox-Analyzer. The activity of these phosphonate compounds, 3, 6, 8, and 11, as regulators of Hb O2 affinity was evaluated on highly purified human Hb. The O2 affinities of these Hbs ranged from somewhat greater than to lower than that of unmodified, human Hb (control 12.1 torr). As shown in , the P50 values range from 8.9 mm Hg for 3 to 12.8 mm Hg for 8. Values for the Hill coefficient, which represents the cooperative effect of O2 binding to the four globin subunits in the Hb tetramer, are in the range of 1.5–2.6 compared to the control (cell-free Hb) with a value of 2.5. As seen in and represented in , these compounds maintained the cooperativity of tetrameric Hb. The most significant and potentially useful results for O2 affinity and cooperativity were obtained using compound 11, which combines covalent and electrostatic interactions to cross-link the β chains.
Table I. O2 Binding properties of the Hb exposed to 2,3-DPG analogs.
Hb modified with compound 3 exhibited an O2 affinity higher than that of unmodified Hb, but it is important to note that it is still considered to be in a range capable of delivering O2 to tissues (Macdonald and Winslow Citation1992). A new generation of chemically modified Hbs with even lower P50s (i.e. high O2 affinity) and decreased cooperativity are under development as O2 therapeutics based on the idea that they may enhance targeted delivery of O2 to the tissues that need it most (Tsai et al. Citation2003, Wettstein et al. Citation2003, Winslow Citation2003). Compound 6 exhibited an O2 affinity and cooperativity similar to the control of cell-free Hb. The n value of the Hb modified with compound 8, is lower than that of unmodified Hb. One possible explanation for this may be by the presence of the aromatic rigid structure which might reduce movement of the protein that is vital for cooperativity of the subunits.
Conclusion
In general, Hb modified with these phosphonate anions maintained cooperativity of the subunits. With the exception of compound 3, all of them have exhibited low O2 affinity compared to the Hb control, which is paramount for O2 transport. Compound 11 yielded the most favorable result, since it maintained cooperativity while decreasing O2 affinity. Based on this result and other literature references we deduced that electrostatic interactions alone may not be enough to give the desired interaction. It may be necessary to establish a covalent interaction between hemoglobin and the allosteric effector. In conclusion, these results show that the phosphonate analogs investigated in this study represent compounds that can regulate the O2 affinity of Hb.
Acknowledgements
We are grateful to the Howard University Graduate School (HUGS) for financial support of TWK. Accurate mass data were obtained on a spectrometer purchased with funds from NSF (CHE-1126533).
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by National Institutes of Health grants R01HL078840 and R01DK070862 to AFP.
References
- Astrup P, Rorth M. 1971. Oxygen Affinity of Hemoglobin and Red Cell Acid Base Status. Academic Press: New York.
- Bakker JC, Berbers GA, Bleeker WK, den Boer PJ, Biessels PT. 1992. Preparation and characterization of cross linked and polymerized hemoglobin solutions. Biomater Artif Cells Immobilization Biotechnol. 20:233–241.
- Berkowitz DB, Sloss DG. 1995. J Org Chem. 60:7047–7050.
- Blackburn GM, Kent DE. 1981. A novel synthesis of α- and γ- fluoroalkylphosphonates. J Chem Soc Chem Commun. 11:511–513.
- Blackburn GM. 1981. Chem Ind (London). 5:134–138.
- Burke TR Jr.,Smyth MS, Nomizu M, Otaka A, Roller PP. J1993. Org Chem. 58:1336–1340.
- Caplan NA, Pogson CI, Hayes DJ, Blackburn GM. 1998. Bioorg Med Chem Lett. 8:515–520.
- Chambers RD, O’Hagan D, Lamont RB, Jain SC. 1990. The difluoromethylenephosphonate moiety as a phosphate mimic: Xray structure of 2-amino-1,1-difluoroethylphosphonic acid. Chem Commun. 15:1053–1054.
- Engel R. 1977. Chem Rev. 77:349–367.
- Gould SA, Sehgal LR, Sehgal HL, Moss GS. 1992. Artificial blood: current status of hemoglobin solutions. Crit Care Clin. 8:293–309.
- Horton H, Moran L, Ochs R, Rawn J, Scrimgeour K. 1993. Principles of Biochemistry. New Jersey: Neil Patterson.
- Kamber M, Just G. 1985. Can J Chem. 63:825.
- Kennedy J. 1957. Patent Brit 778,077. Chem Abstr. 51:17980b.
- Macdonald VW, Winslow RM. 1992. Oxygen Delivery and myocardial function in rabbit hearts perfused with cell-free hemoglobin. J Appl Physiol72:476–483.
- Moonen K, Laureyn I, Stevens CV. 2004. Chem Rev. 104:6177.
- Palacios F, Alonso C, de los Santos JM. 2005. Chem Rev. 105:899.
- Palmer AF, Sun G, Harris DR. 2009. Tangential flow filtration of hemoglobin. Biotechnol Prog. 25:189–199.
- Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. 1996. The risk of transfusion-transmitted viral infections. The retrovirus epidemiology donor study. N Engl J Med. 334:1685–1690.
- Spahn DR. 2000. Current status of artificial oxygen carriers. Adv Drug Deliv Rev. 40:143–151.
- Stremler KE, Poulter CDJ. 1987. Am Chem Soc. 109:5542–5544 (geranyl diphosphate analogs as FDP synthase pseudo-substrates).
- Thatcher GRJ, Campbell AS. 1993. J Org Chem. 58:2272–2281.
- Tozer MJ, Herpin TF. 1996. Tetrahedron. 52:8619–8683.
- Tsai A, Vandergriff KD, Intalietta M, Winslow RM. 2003. Targeted O2 delivery by low-P50 hemoglobin: a new basis for O2 therapeutics. Am J Physiol Heart Cric Physiol. 285:H1411–H1419.
- Waschbüsch R, Carran J, Marinetti A, Savignac P. 1997. Chem Rev. 97:3401.
- Wettstein R, Tsai AG, Winslow RM. 2003. Resuscitation with poly (ethylene glycol)-modified human hemoglobin improves microcirculatory blood flow and tissue oxygenation after hemorrhagic shock in awake hamsters. Crit Care Med. 31:1824–1830.
- Wiemer DF. 1997. Tetrahedron. 53:16609.
- Winslow RM. 1995. Blood substitutes, a moving target. Nat Med. 1: 1212–1215.
- Winslow RM. 2003. Current status of blood substitute research towards a new paradigm. J Intern Med. 253:508–517.