Abstract
IL-27 plays an important role in anti-cancer activity. The -964A/G polymorphism in IL-27 gene has been implicated in susceptibility to cancer, but the results were conflicting. The aim of this study was to assess the association between this polymorphism and cancer risk. Pubmed and Wanfang database were searched for all publications concerning IL-27 -964A/G polymorphism and cancer risk. Odds ratio (OR) and 95% confidence interval (CI) were used to assess the strength of association. Statistical analysis was performed using Stata 11.0 software. A total of eight case–control studies including 2044 cancer cases and 2197 controls were identified. Overall, significant association between IL-27 -964A/G polymorphism and cancer risk was observed (GG versus AA: OR = 1.26, 95% CI = 1.03–1.52; GG versus AG + AA: OR = 1.20, 95% CI = 1.00–1.44). In subgroup analysis based on cancer type, significant association was found in colorectal cancer (GG versus AA: OR = 1.55, 95% CI = 1.07–2.27; AG versus AA: OR = 1.31, 95% CI = 1.02–1.67). The current meta-analysis suggests that IL-27 -964A/G polymorphism might enhance cancer risk. However, large-scale and well-designed studies are still needed to confirm the result of our meta-analysis. The association of IL-27 polymorphism with colorectal cancer may provide insight for future therapies.
Introduction
Cancer remains a critical public health problem around the world. It is one of the leading mortality in the world and the worldwide cancer burden continues to increase (Citation1). Although the exact mechanism of carcinogenesis remains poorly understood, susceptibility genes combining with environmental triggers may play an important role (Citation2). Many genetic factors have been identified, among which single nucleotide polymorphisms (SNPs) have been investigated widely, which could alter cancer predisposition (Citation3), suggesting that detection of genetic risk factors may provide further diagnostic capabilities.
Interleukin-27 (IL-27), a heterodimeric cytokine, is a new identified member of interleukin-12 (IL-12) family. This molecule is formed by dimerization of two subunits. One subunit is a soluble type I cytokine receptor-like molecule which is coded by the Epstein–Barr virus induced gene 3 (EBI3) and has sequence homology with IL-12p40 (Citation4). Another subunit is IL27p28, an IL-12p35-related polypeptide, belongs to the family of long-chain four-helix bundles cytokines (Citation5). IL-27p28 gene has been mapped to chromosome 16p11, containing five exons separated by four introns. The -964A/G polymorphism (rs153109) of IL-27p28 gene promoter was first identified in a case–control study and showed a relationship with asthma susceptibility (Citation6). After that, several case–control studies were conducted to investigate the association between IL-27p28 gene -964A/G polymorphism and cancer risk in Chinese population (Citation7–14). However, conflicting results were observed, which might be caused by small sample size of each individual study. In order to clarify the association between IL-27 gene -964A/G polymorphism and cancer risk, we conducted a comprehensive meta-analysis of all eligible case–control studies.
Materials and methods
Search strategy for relationship between IL-27 rs153109 polymorphism and cancer risk
A systemic literature search was conducted in the Pubmed and Wanfang database, using the following keywords: (“interleukin-27” or “IL-27”) and (“polymorphism” or “genotype” or “variant” or “mutation”) and (“cancer” or “carcinoma” or “tumor” or “neoplasm” or “malignancy”) without language restriction. In addition, the reference lists of all eligible studies were checked for potential studies. The search was updated on 18 May 2014.
Selection criteria
The indentified articles should meet the following criteria: (Citation1) investigated IL-27 gene polymorphism and cancer risk in Chinese population; (Citation2) used a case–control design; and (Citation3) had an odds ratio (OR) with 95% confidence interval (CI) or provided sufficient data for estimating OR with 95% CI. The exclusion reasons: (Citation1) studies based on families or siblings; (Citation2) did not consisted with Hardy–Weinberg equilibrium (HWE) in the control population; (Citation3) duplicated of previous studies; and (Citation4) studies without detail genotype frequencies, which were unable to calculate OR.
Data extraction
Two authors (Xiu-Peng Xu and Hong-Lu Chao) independently reviewed and extracted data from selected articles and reached a consensus on every item. The information collected from each publication included the first author’s name, publication year, country, source of controls, genotyping methods, sample size of cases and controls, genotype distributions of cases and controls and p value for HWE.
Statistical analysis
ORs and 95% CI were calculated to assess the strength of the association between IL-27 gene -964A/G polymorphism and cancer risk. The estimates of pooled ORs were achieved by calculating a weighted average of OR from each study. The significance of pooled OR was determined by Z test, and the p value less than 0.05 was considered significant. Five genetic models were assessed: allele model (G versus A), homozygote model (GG versus AA), heterozygote model (AG versus AA), dominant model (AG + GG versus AA) and recessive model (GG versus AG +AA), respectively. Stratified analysis was also conducted based on cancer types, which limited to colorectal cancer and nasopharyngeal cancer.
Chi-square-based Q test was conducted to measure the heterogeneity between eligible studies. If p value was less than 0.05, then the heterogeneity was considered statistical significant, and then the random effects model (based on Dersimonian–Laird method) was used (Citation15). Otherwise, the fixed effects model (based on Mantel–Haenszel method) was conducted (Citation16). Sensitivity analysis was performed by omitting each study in turn to assess the results stability. Additionally, potential publication bias were evaluated quantitatively by performing funnel plots and assessed quantitatively by Begg’s test and Egger’s test (Citation17,Citation18). All statistical tests for this meta-analysis were conducted using Stata 11.0 software (Stata Corporation, College Station, TX).
Results
Study characteristics
The detailed selection process of this study was shown in . A total of 52 publications from Pubmed and Wanfang database were reviewed. Through screening the titles or abstracts, 26 articles were excluded for obviously irrelevant. After reading full-text articles, 18 articles were excluded for exploring other genetic polymorphisms and cancer risk. Manual search of references cited in the published literatures did not reveal any additional articles. Finally, eight articles with 2044 cases and 2197 controls were included in this meta-analysis. The main characteristics of included studies are summarized in . Among these included studies, six were published in English and two were published in Chinese. All cancer cases included in this meta-analysis were pathologically or histologically confirmed, and all controls were selected from healthy or non-cancer populations. In addition, the genotype distributions in controls were in agreement with HWE (p > 0.05).
Table 1. Characteristics of all eligible studies.
Meta-analysis results
The main results of this meta-analysis were shown in . In the overall analysis, we found a significant association between rs153109 polymorphism and cancer risk in the homozygous model (GG versus AA: OR = 1.26, 95% CI = 1.03–1.52) () and recessive model (GG versus AG + AA: OR = 1.20, 95% CI = 1.00–1.44). In the subgroup analysis by cancer type, significant association between rs153109 polymorphism and increased colorectal cancer risk was detected in the homozygous model (GG versus AA: OR = 1.55, 95% CI = 1.07–2.27) () and heterozygous model (AG versus AA: OR = 1.31, 95% CI = 1.02–1.67). However, no significant association was found in nasopharyngeal carcinoma in all comparison models.
Figure 2. Forest plot of the IL-27 -964A/G polymorphism and cancer risk using fixed-effect model (homozygous model GG versus AA): subgroup analysis by cancer type. CRC colorectal cancer; NPC nasopharyngeal carcinoma; Other esophageal cancer, glioma, hepatocellular carcinoma, epithelial ovarian cancer, respectively.
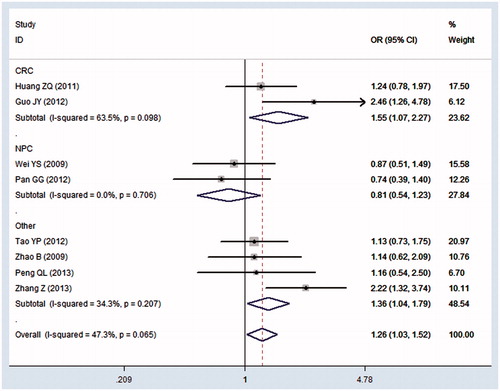
Table 2. Meta-analysis results of IL-27 rs153109 polymorphism and cancer risk.
Test of heterogeneity
As shown in , significant heterogeneity was identified in the allele model (G versus A: Pc = 0.007, I2 = 63.7%) and dominant model (GG + AG versus AA: Pc = 0.013, I2 = 60.6%). Therefore, random-effects model was chosen to generate wider CIs in these two genetic models. When stratified by cancer type, the heterogeneity still exists in the allele model (G versus A: Pc = 0.044, I2 = 75.7%) and dominant model (GG + AG versus AA: Pc = 0.060, I2 = 71.7%) in colorectal cancer. However, the heterogeneity in nasopharyngeal carcinoma disappeared in all comparison models.
Sensitivity analysis
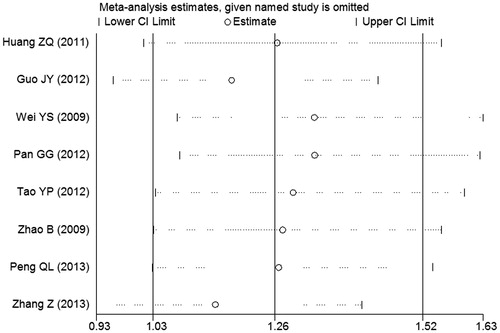
To assess the influence of each single study on the pooled OR, sensitivity analysis was performed by omitting one study each time. And the results suggested that omission of any individual study made no significant difference for all comparison models, suggesting that our results were statistically robust ().
Publication bias
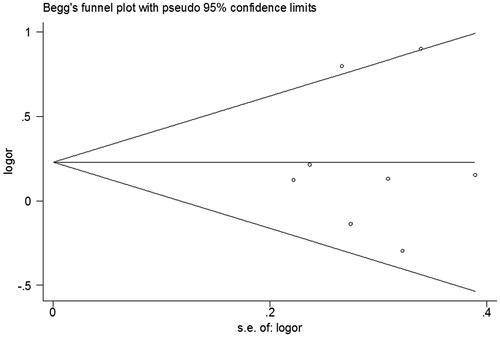
Begg’s funnel plots and Egger’s test were conducted to assess the publication bias of the currently available literature. The shapes of Begg’s funnel plots did not reveal any evidence of obvious asymmetry (). In addition, the results of Egger’s test confirmed no significant publication bias existing in our study.
Figure 3. The sensitivity analysis of IL-27 -964A/G polymorphism with cancer risk (homozygous model GG versus AA). The middle vertical axis indicates the overall OR, and the two vertical axes indicate its 95% confidence interval (CI). Every hollow round indicates the pooled OR when the left study was omitted in this meta-analysis.
Discussion
IL-27 is produced early after activation by antigen-presenting cells, including monocyte-derived dendritic cells and lipopolysaccharide-stimulated monocytes (Citation19). IL-27 mediates its biologic functions via a heterodimeric receptor constituted by WSX-1/TCCR and glycoprotein 130 (gp130) subunits (Citation20). IL-27 engages the receptor and activates Janus kinase (JAK)-signal transducer and activator of transcription (STAT) and mitogen-activated protein kinase (MAPK) signaling (Citation21). IL-27 synergizes with IL-12 to potentiate IFN-γ production by activated naïve T-cell and natural killer-cell populations (Citation22). Therefore, IL-27 is thought to promote Th1 polarization. All these functional characteristics of IL-27 indicate that it might play an important role in anti-cancer activity. In addition, transgenic over-expression of IL-27 leads to anticancer activity in colon cancer and lymphoma, which is mainly mediated through CD8+ T cells with enhanced cytotoxic T lymphocyte (CTL) activity (Citation23,Citation24). Thus, we postulated that SNPs of IL-27 may influence the anti-cancer activity of IL-27, which might account for etiology of cancer.
Another anti-cancer mechanism of IL-27 may involve IL-10. IL-27 can stimulate the production of IL-10 in CD8+ T cells which contributes to tumor rejection (Citation25). Targeted disruption of IL-10 gene in mice caused spontaneous colitis and overt risk for colorectal cancer (Citation26), suggesting IL-10 could be potential targets for colorectal cancer therapy.
IL-27 and IL-10 have been proposed as therapies to promote anti-tumor responses. Recently, a mathematic model was published to calculate the amount of IL-27 that needs to be injected in mice to reject the tumor (Citation27). One may deliver IL-27 or IL-10 to colon cancer via yeast that is programmed to express IL-27 or IL-10, or infect tumor cells with virus that are designed to produce IL-27 and thus generate tumor rejection (Citation27). Other therapies may involve modification of IL-27 receptor or JAK/STAT1 pathway. SNPs of IL-27 in colorectal cancer may be used as a biomarker or guide the therapies in a selected group of patients (Citation28).
In the present study, we conducted a comprehensive meta-analysis to explore IL-27 gene rs153109 polymorphism and cancer risk based on the eight case–control studies, including 2044 cancer cases and 2197 controls. The present meta-analysis provides evidence that IL-27 -964A/G polymorphism was overall associated with cancer risk under homozygous model and recessive model. When stratified by cancer type, significant association was found in colorectal cancer under homozygous model and heterozygous model. However, no significant association with nasopharyngeal carcinoma was observed in any of the five comparison models. Considering the limitation participants of the included case–control studies for each cancer type, the results should be explained with caution.
The heterogeneity test is an important part of a sound meta-analysis. In this meta-analysis, significant heterogeneity was detected in allele model and recessive model, but no significant heterogeneity was found in homozygous, heterozygous, and dominant models. When stratified by cancer type, similar results were found in colorectal cancer. However, the heterogeneity disappeared in nasopharyngeal carcinoma in all comparison models. The heterogeneity might attribute to difference in age, gender, living environment and life styles. Therefore, the precise relationship of IL-27 -964A/G polymorphism and cancer risk should be investigated in larger sample size case–control studies. In addition, sensitivity analysis was performed to estimate the effect of each individual study, and the results indicated that our meta-analysis was not influenced by any single study. Furthermore, no publication bias was detected, which showed that our results were robust and reliable.
To the best of our knowledge, this is the first meta-analysis investigated the association between IL-27 -964A/G polymorphism and cancer risk. However, several limitations of our meta-analysis should be noted. First, the meta-analysis results should be interpreted with caution because of the small sample size. Second, only articles included in Pubmed and Wanfang database were searched, and some studies might be left out. Third, our meta-analysis was based on unadjusted estimates, while a more precise analysis should be performed on individual data such as, gender, age, smoking status, drinking status, obesity, environmental factors and other lifestyle.
In conclusion, our meta-analysis suggested that IL-27 -964A/G polymorphism enhanced cancer susceptibility in Chinese population. In addition, our results also demonstrated that IL-27 -964A/G polymorphism increased colorectal cancer risk. However, large and well-designed studies based on homogeneous cancer patients are needed to confirm our findings.
Declaration of interest
The authors declare no conflicts of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA-Cancer J Clin 2011;61:69–90
- Pharoah PD, Dunning AM, Ponder BA, Easton DF. Association studies for finding cancer-susceptibility genetic variants. Nature Rev Cancer 2004;4:850–60
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74
- Devergne O, Hummel M, Koeppen H, et al. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J Virol 1996;70:1143–53
- Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 2002;16:779–90
- Chae SC, Li CS, Kim KM, et al. Identification of polymorphisms in human interleukin-27 and their association with asthma in a Korean population. J Human Genet 2007;52:355–61
- Huang ZQ, Wang JL, Pan GG, Wei YS. Association of single nucleotide polymorphisms in IL-12 and IL-27 genes with colorectal cancer risk. Clin Biochem 2012;45:54–9
- Peng Q, Qin X, He Y, et al. Association of IL27 gene polymorphisms and HBV-related hepatocellular carcinoma risk in a Chinese population. Infect Genet Evol 2013;16:1–4
- Tao YP, Wang WL, Li SY, et al. Associations between polymorphisms in IL-12A, IL-12B, IL-12Rbeta1, IL-27 gene and serum levels of IL-12p40, IL-27p28 with esophageal cancer. J Cancer Res Clin Oncol 2012;138:1891–900
- Wei YS, Lan Y, Luo B, et al. Association of variants in the interleukin-27 and interleukin-12 gene with nasopharyngeal carcinoma. Mol Carcinog 2009;48:751–7
- Zhang Z, Zhou B, Wu Y, et al. Prognostic value of IL-27 polymorphisms and the susceptibility to epithelial ovarian cancer in a Chinese population. Immunogenetics 2014;66:85–92
- Zhao B, Meng LQ, Huang HN, et al. A novel functional polymorphism, 16974 A/C, in the interleukin-12-3′ untranslated region is associated with risk of glioma. DNA Cell Biol 2009;28:335–41
- Junyu G, Anqiang Q, Rukun L, et al. Association of interlenkin-27 gene polymorphism with genetic susceptibility to colorectal cancer. Chongqing Med 2012;41:948–50
- Guogang P, Dong L, Lina L, Chunchuan H. Association of the genotype and serum level of IL-27 with nasopharyngeal carcinoma. Shandong Med J 2012;52:18–20
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research Ed) 1997;315:629–34
- Owaki T, Asakawa M, Morishima N, et al. A role for IL-27 in early regulation of Th1 differentiation. J Immunol 2005;175:2191–200
- Holscher C, Holscher A, Ruckerl D, et al. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol 2005;174:3534–44
- Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol 2007;25:221–42
- Cordoba-Rodriguez R, Frucht DM. IL-23 and IL-27: new members of the growing family of IL-12-related cytokines with important implications for therapeutics. Expert opinion on biological therapy 2003;3:715–23
- Chiyo M, Shimozato O, Yu L, et al. Expression of IL-27 in murine carcinoma cells produces antitumor effects and induces protective immunity in inoculated host animals. Int J Cancer 2005;115:437–42
- Cocco C, Di Carlo E, Zupo S, et al. Complementary IL-23 and IL-27 anti-tumor activities cause strong inhibition of human follicular and diffuse large B-cell lymphoma growth in vivo. Leukemia 2012;26:1365–74
- Liu Z, Liu JQ, Talebian F, et al. IL-27 enhances the survival of tumor antigen-specific CD8+ T cells and programs them into IL-10-producing, memory precursor-like effector cells. Eur J Immunol 2013;43:468–79
- Berg D, Davidson N, Kühn R, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest 1996;98:1010–20
- Liao KL, Bai XF, Friedman A. Mathematical modeling of interleukin-27 induction of anti-tumor t cells response. PLoS One 2014;9:e91844
- Hunter CA, Kastelein R. Fifteen years of interleukin-27 – discovery, advances and translation. Immunity 2012;37:960–9