Abstract
Objective. Severe hemorrhage is a leading cause of death and difficult to control even by trained medical personnel. Current interventions have significant limitations in the prehospital setting; therefore, a need exists for a new and effective treatment. iTraumaCare has designed a temporary wound closure device, the iTClamp, which controls external hemorrhage from open wounds within compressible zones. The device approximates the wound edges, sealing the skin within a pressure bar, enabling creation of a hematoma and subsequent clot formation. The objective of this study is to test the effectiveness of the iTClamp to control external bleeding due to a major vascular injury to the groin in an in vivo swine model. Methods. Twenty Yorkshire-cross male swine were enrolled in this study. A complex groin injury was created by complete excision of the femoral artery and vein along with some surrounding muscle. The animals were divided into four treatment groups: control (no treatment), early iTClamp treatment, late iTClamp treatment, and standard gauze treatment. Survival rate, survival time, and blood loss were the primary endpoints. Physiologic parameters (heart rate, blood pressure, oxygen saturation) were monitored throughout the experiment and blood samples were collected to analyze partial thromboplastin time and fibrinogen. Results: All (100%) of the animals treated with the iTClamp lived through the end of the experiment, compared to 60% in standard gauze treated and 0% of untreated control animals (early and late iTClamp vs. control and standard gauze, Fisher's exact, p = 0.003). Both the early iTClamp and late iTClamp treatment groups survived significantly longer than the untreated control pigs (Mann-Whitney U-test, p < 0.009). External blood loss was significantly lower in animals treated with the iTClamp (early) compared to no treatment (Mann-Whitney U-test, p < 0.008). There was no significant change in physiologic or hematologic parameters between treatment groups. Conclusions: The iTClamp showed statistically significant improvement in survival, survival time, and estimated blood loss when compared to no treatment. This proof-of-concept study demonstrates the potential of the iTClamp to control severe bleeding and prevent blood loss.
Introduction
Trauma is a leading cause of mortality worldwide.Citation1,2 Uncontrolled hemorrhage is the second leading cause of early deaths among trauma patients, with only central nervous system (CNS) injury consistently being more lethal.Citation3–5 Early control of hemorrhage is critical to the survival of trauma patients in both military and civilian settings.Citation3,4,Citation6–8 In the prehospital period, hemorrhage contributes to 33–56% of civilian trauma-related deaths and in recent military operations uncontrolled hemorrhage was the primary cause of potentially survivable battlefield death.Citation3,Citation8–13 Due to the critical need for early and effective control of hemorrhage, significant research has been invested in new technologies to control hemorrhage, such as the development of hemostatic agents and use of tranexamic acid (TXA).
Retrospective studies have shown the efficacy of hemostatic agents in both militaryCitation14,15 and civilian settings.Citation16 However, recent studies have indicated that in swine exsanguination models hemostatic dressings are not superior to standard gauze when applied under pressure.Citation17,18 In addition, there are safety concerns with hemostatic agents; for example, zeolite-based agents generate an exothermic reaction, which can cause burns, and nonbiodegradable agents can be cumbersome to remove from wounds and may embolize.Citation14,Citation19–22 Furthermore, hemostatic agents are limited by the requirement for 3 minutes or more of manual pressure following wound packing.
The CRASH-2 trial demonstrated that early treatment (within 3 hours) of patients with TXA leads to a significant reduction in all cause mortality.Citation23,24 As a result, TXA treatment is now being incorporated into tactical combat casualty care guidelines.Citation25 However, TXA will not stop hemorrhage from large vessels in junctional (axilla, groin, neck) regions. Groin hemorrhage is the most common type of junctional hemorrhage.Citation7,Citation9 Even with extensive training and access to new technologies, most first responders, medics, and soldiers are still challenged to control this type of severe bleeding in the prehospital setting.Citation3,Citation9 Junctional hemorrhage, which is not amenable to tourniquets, is particularly difficult to control and recently has received significant research.Citation26 It is a significant cause of potentially survivable death among Canadian and US soldiers; and recent estimates suggest that up to 4.6% of causalities during recent conflicts could have been saved with a device that effectively controlled junctional hemorrhage.Citation7,Citation27 To date, only two products, the Combat Ready Clamp and Junctional Emergency Treatment Tool, have received approval by the US Food and Drug Administration for control of junctional bleeding; however, these devices are limited to use only in inguinal areas, require application by trained medical personnel, completely occlude distal and collateral blood flow, and make it difficult to transport a patient.Citation26,Citation28 Given the limitations of current interventions, additional tools are needed to control hemorrhage in the prehospital setting. Ideally, a new treatment will be effective, safe, quickly applied, and easily removed, and will require minimal training for use.Citation26
iTraumaCare Inc. has designed a temporary wound closure device, the iTClamp, to control severe hemorrhage from open wounds within compressible zones. The device seals the skin edges within a pressure bar, enabling creation of a hematoma where blood collects under pressure to form a stable clot until definitive surgical repair. It is applied by aligning the device to the wound edges and then pressing the arms together to close the device; a locking mechanism prevents the device from opening. Suture needles position the skin edges between the pressure bars to form a complete seal and anchor the device to reduce slippage. It can be easily removed and is intended to be a temporary solution until surgical repair.
This study was designed to test the potential effectiveness of the iTClamp to control external bleeding due to major vascular injury in the groin. For this purpose, a swine model for rapid exsanguination involving a groin injury with excision of a segment of the femoral artery, vein, and a portion of the overlying muscles was used. This model was chosen as it has been previously established to study the effectiveness of hemorrhage control treatments, including hemostatic agents, and permits creation of a cavitary injury better simulating complex injuries.Citation15,Citation17,Citation18,Citation20,Citation29–35
Methods
Experimental Design
Animal ethics approval was obtained by animal care committees at the University of Alberta and Innovotech, Inc. (Edmonton, Alberta). All presurgical handling and preparation of the animals were performed in compliance with the animal care guidelines established by the Canadian Council on Animal Care.Citation36 Throughout the experiment a team of veterinarians and veterinary technicians monitored the animals. Twenty 20- to 25-kg Yorkshire-cross large white castrated male swine were enrolled in the study. All animals were subject to a junctional injury of the femoral vascular complex, including the surrounding muscle, creating a cavitary defect. Following injury, 5 received no treatment, 5 were treated with the iTClamp device 10 s after injury, 5 were treated with the iTClamp after a 3-min delay, and 5 were treated with standard gauze after a 3-min delay. A 3-min delay was chosen to simulate field conditions, similar to previous studies.Citation29,Citation33 This experiment was limited to 5 animals per treatment group since a power analysis indicated only 5 animals were required to provide statistical relevance and would reduce the number of animals used (α = 0.05, β = 0.20, power = 0.89).
Outcomes
The primary endpoints measured were survival, survival time, and external blood loss. Secondary endpoints measured were vital signs (heart rate, systolic blood pressure, and oxygen saturation), partial thromboplastin time (PTT), and fibrinogen. Wound size and histologic changes of the skin surrounding the wounds were also examined at necropsy to see size of the cavity and to determine if application of the iTClamp resulted in additional injury.
Procedure
The animals were fasted for 12–18 hours prior to injury with free access to water. On the day of the surgery the animals were medicated with 10 mg/kg ketamine, 2 mg/kg butorphanol, and 80 μg/kg medetomidine. Anesthesia was maintained with medetomidine (10 μg/kg/hr) and ketamine (5 mg/kg/hr)Citation37; additional replacement of fluids due to blood loss was not part of this experiment. The animals were allowed to breathe room air spontaneously. For the spinal anesthetic the animals were injected with 4 mL of 1% plain lidocaine at the lumbosacral junction. The effectiveness of the spinal anesthesia was ensured prior to the surgical procedure with testing for sphincter tone and lower limb toe pinch. Test subjects were placed in the supine position (with the exception of the first control subject; see Results) to ensure free bleeding from the wound.
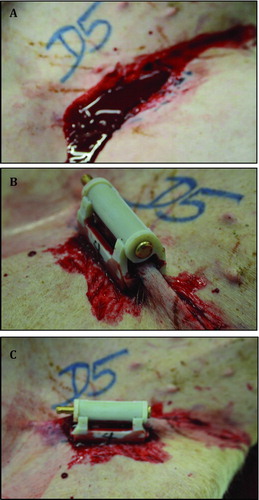
To induce injury, the femoral pulse was palpated and a 4.5-cm incision with a scalpel was made through the skin and proximal thigh soft tissue over the pulse. Overlying quadriceps and abductor muscles were excised to expose the proximal femoral artery and vein. The femoral artery and vein were grasped in a clamp, and then a 2-cm section of tissue including the artery and vein was excised below the inguinal ligament. The vessels were not stretched, but excised in situ to decrease the risk of the vessels spontaneously contracting. This was confirmed by visually examining the removed vessels. Timing started upon the observation of free bleeding from the wound. illustrates the groin injury before and after application of the iTClamp. The subjects were randomly assigned to each study group.
Figure 1. Complex groin injury and application of treatment. (A) A complex and lethal groin injury was created by complete excision of a 2- to 3-cm section of the femoral artery and vein below the inguinal ligament. (B, C) Approximation of the wound edges with ITClamp seals the skin within the pressure bars of the device.
Control: No treatment was initiated.
Early iTClamp: After 10 seconds the overlying skin in the early iTClamp treatment group was closed with the device. No additional treatment was performed on this group.
Late iTClamp: After 3 minutes the overlying skin in the late iTClamp treatment group was closed with the device. No additional treatment was performed.
Standard gauze: After 3 minutes the wound was packed with standard gauze followed by manual pressure for an additional 3 minutes. At 3 minutes (6 min from injury), the pressure was stopped without removing the gauze. No additional treatment was performed.
An individual with no previous medical training applied the iTClamp to early and late iTClamp treatment groups (5–10 sec to apply); experienced trauma surgeons performed the wound packing with standard gauze and applied direct manual pressure in the standard gauze treatment group. Animals were monitored for 180 minutes under anesthesia or until they expired. Animals that survived until 180 minutes were euthanized with euthanyl > 150 mg/kg.
Data Collection
Heart rate, blood pressure, and blood saturation were monitored throughout the experiment and recorded at baseline, every 15 minutes for the first hour and every hour until 180 minutes or termination. Heart rate was recorded from a heart rate monitor, blood pressure was recorded from a cuff sphygmomanometer, and blood oxygen saturation was recorded from a pulse oximeter (placed on the ear). Blood samples for coagulation profile (PTT, fibrinogen) were also collected in the same time schedule.
External blood loss was measured by weighing the blood-soaked absorbent pads and gauze, and subtracting from the dry weight of the pads and gauze. Absorbent pads were placed underneath the animal such that they did not have direct contact with the wound or interfere with free flow of blood from the wound. At necropsy the iTClamps, gauze, blood clots, and/or remaining blood were removed from the wounds. The sizes of the wounds were measured (width, length, and depth) and the thickness of distal and proximal skin was recorded. Skin tissue samples proximal to the wound were harvested for histological examination. Histological examination was completed by a veterinary pathologist at Prairie Diagnostic Services (Saskatoon, Saskatchewan, Canada); the pathologist was blinded to the treatment groups.
Table 1. Treatment with iTClamp Increases Survival and Survival Time (Mean ± Standard Deviation)
Statistical Analysis
All experimental values are expressed as means ± standard deviation. Survival data were analyzed by Fisher's exact test, and survival time and external blood loss were analyzed using a Kruskal-Wallis nonparametric test or Mann Whitney U-test. A two-tailed p-value < 0.05 was considered statistically significant.
Results
This study was designed to investigate the effectiveness of the iTClamp to control junctional groin bleeding due to major vascular injury in the rapid exsanguination swine model. Survival was significantly higher in both the early iTClamp and late iTClamp treatment groups; 100% of the animals in both iTClamp-treated groups lived to the end of the experiment compared to 0% of the control group; survival in the standard gauze group was 60% (both iTClamp treatment groups vs. control and standard gauze, Fisher's exact, p = 0.003; ). The survival time in the early iTClamp (mean > 180 min) and late iTClamp (mean > 180 min) treatment groups was significantly longer than that of the control group (mean = 38.4; ; Mann-Whitney U-test, p < 0.009). The mean survival time of the standard gauze-treated group was 139 minutes. A nonparametric Kruskal-Wallis test showed significant differences in survival time between all four treatment groups (p < 0.02).
There was a single outlier in the control group. The first control subject was placed in a left lateral decubitus position, which allowed the leg to place pressure on the site of injury and slow the bleeding. This control was then placed into a supine position to allow free bleeding to occur without interference. This resulted in an outlying value for survival time in this subject. All other test subjects were placed in the supine position from the start.
Analysis was performed for external blood loss of all pigs treated under each condition. The mean external blood loss was significantly lower in animals treated with the iTClamp (early treatment mean = 0.12 kg; late treatment mean = 0.48 kg) compared to control animals (mean = 1.06 kg, Mann-Whitney U-test, p < 0.009 for both iTClamp treatment groups, ). No statistical difference in external blood loss was observed in late iTClamp treatment versus standard gauze treatment (mean = 0.68 kg); however, early iTClamp treatment resulted in a statistically significant decrease in external blood loss compared to standard gauze treatment (Mann-Whitney U-test, p = 0.008). A nonparametric Kruskal-Wallis test showed significance in external blood loss for all four treatment groups (p < 0.002).
Figure 2. External blood losses. External blood loss was determined by weighing collection pads, measured in kilograms. Error bars represent standard deviation. A nonparametric Kruskal-Wallis test showed significance in external blood loss for all four treatment groups (p < 0.002).
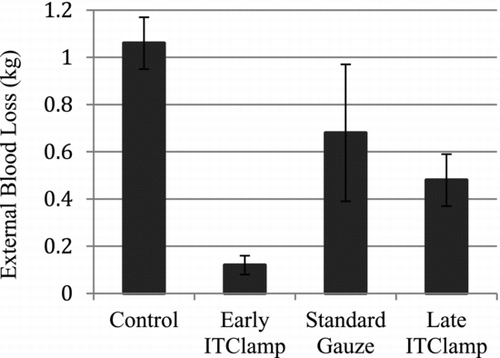
Figure 3. No significant change in physiological parameters between treatment groups. (A) Heart rate; measured in beats per minute (bpm). (B) Systolic blood pressure. (C) oxygen saturation (SP2O2). Error bars represent standard deviation. Mean of survivors only. •, Control; ×, early ITClamp; ▴, standard gauze; ░, late iTClamp.
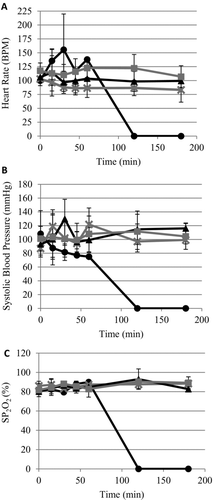
Table 2. Wound Measurements at Necropsy
The baseline hemodynamic and hematological parameters measured before vascular injuries were within normal ranges and not different between treatment groups. Following injury, heart rate and blood pressure were altered in each treatment arm; however, there was no significant difference observed between treatment groups (, B). There was no significant change observed in oxygen saturation during the experiment (). Similarly, there was no significant change in mean PTT or mean fibrinogen clotting time between treatment groups (data not shown).
Following euthanasia, the blood clot was removed from the wounds to check the status of injury and the status of the clot. Upon manually spreading the wound open at the site of injury, the clot was visibly very stable. After removal of the blood clot and/or gauze from the wound at necropsy, the wound dimensions and skin thickness were measured to determine if iTClamp treatment affected wound formation. No statistical difference was observed in the wound dimensions or skin thickness ().
Skin tissue samples at necropsy were harvested for histologic examination. No histologic changes in the epidermal or dermal layers surrounding each wound (lesions, inflammation, bruising, or neutrophil infiltration) were observed (data not shown).
Discussion
At the point-of-injury in a military setting, any casualty with severe external bleeding is likely to be in a hostile environment where there is risk of additional injuries to both the casualty and the care provider.Citation38 Without the immediate ability to control hemorrhage, exsanguination is a significant risk. Early and effective control of junctional hemorrhage in the prehospital setting can potentially save many lives.Citation7,Citation27,Citation39 iTraumaCare has developed a novel medical device, the iTClamp, which is designed to control bleeding at the point-of-injury (within seconds) in the prehospital, hospital, or tactical setting. This study evaluated the short-term efficacy of using the iTClamp to control external junctional bleeding with major vascular injuries in rapid exsanguination swine model.
Early and late applications of the iTClamp resulted in significantly higher survival rates and time compared to standard gauze treatment and control (). This demonstrates that earlier treatment of hemorrhage is possible and efficacious. As expected, external blood losses were highest in the control group and standard gauze treatment groups (). Sealing the skin allowed less blood to escape from the wound and be measured externally. Pretreatment delays as short as 3 minutes increased the external blood losses of animals treated under the standard gauze and late iTClamp conditions.
The results for improvement in survival, survival time, and external blood loss were statistically significant. This study provides proof-of-principle for the use of the iTClamp for rapid treatment arterial junctional bleeding.
Early control of hemorrhage creates a significant advantage by reducing complications caused by treatment delays. Even when there is adequate iv fluid resuscitation, significant blood losses can lead to hypothermia, coagulopathy, and acidosis; these patients are more susceptible to late mortality due to sepsis and multiple organ failure.Citation3,Citation40–43 Studies involving tourniquets demonstrate that successful tourniquet application prior to the onset of shock significant decreases mortality.Citation44,45 The iTClamp provides a method for early control of hemorrhage within a few seconds and may prevent the onset of shock.
This study was limited to the 180-minute observation window and did not address post-application removal of the iTClamp. Also, this study did not address iv replacement of fluids; although not always immediately available prehospital, iv replacement of fluid is common practice in trauma management. This swine model involving complete transection of the femoral artery and vein has been shown to benefit from vascular retraction and studies have demonstrated less rebleeding with the transection model as compared to a puncture model.Citation20,Citation35,Citation46 In this current study, it is unlikely that vascular retraction had a significant role given the 100% mortality in the control group. Neither the transection nor the puncture model is a direct translation to human scenarios and both models are extensively used as research tools; future studies could examine the ability of the iTClamp to control severe bleeding in a puncture model. In addition, future areas for research include studies comparing injury sites other than the groin (scalp, extremities, other junctional areas); use of ragged, laceration injury modelsCitation17; use of a coagulopathic model; comparison with hemostatic agents (i.e., Combat Gauze); and the combined use of wound packing with iTClamp skin closure.
Conclusion
In summary, utilizing a lethal junctional injury animal model, the iTClamp showed statistically significant improvement in survival, survival time, and estimated blood loss when compared with no treatment. Treatment times were reduced from several minutes to a few seconds.
References
- World Health Organziation. 2003 Injury Chart Book: Graphical Overview of the Global Burden of Injuries. Department of Injuries and Violence Prevention, Noncommunicable Diseases and Mental Health Cluster, World Health Organization; 2002.
- McGee K, Krug E, World Health Organization. Injury: A Leading Cause of the Global Burden of Disease, 2000. Department of Injuries and Violence Prevention, Noncommunicable Diseases and Mental Health Cluster, World Health Organization; 2002.
- Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–11.
- Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38(2):185–93.
- Soreide K, Kruger AJ, Vardal AL, Ellingsen CL, Soreide E, Lossius HM. Epidemiology and contemporary patterns of trauma deaths: changing place, similar pace, older face. World J Surg. 2007;31(11):2092–103.
- Acosta JA, Yang JC, Winchell RJ, Simons RK, Fortlage DA, Hollingsworth-Fridlund P, Hoyt DB. Lethal injuries and time to death in a level I trauma center. J Am Coll Surgeons. 1998;186(5):528–33.
- Kelly JF, Ritenour AE, McLaughlin DF, Bagg KA, Apodaca AN, Mallak CT, Pearse L, Lawnick MM, Champion HR, Wade CE, Holcomb JB. Injury severity and causes of death from Operation Iraqi Freedom and Operation Enduring Freedom: 2003–2004 versus 2006. J Trauma. 2008;64(2 Suppl): S21-6; discussion S6-7.
- Kauvar DS, Wade CE. The epidemiology and modern management of traumatic hemorrhage: US and international perspectives. Crit Care. 2005;9 (Suppl 5):S1–9.
- Eastridge BJ, Hardin M, Cantrell J, Oetjen-Gerdes L, Zubko T, Mallak C, Wade CE, Simmons J, Mace J, Mabry R, Bolenbaucher R, Blackbourne LH. Died of wounds on the battlefield: causation and implications for improving combat casualty care. J Trauma. 2011;71(1 Suppl):S4–8.
- Holcomb JB, McMullin NR, Pearse L, Caruso J, Wade CE, Oetjen-Gerdes L, Champion HR, Lawnick M, Farr W, Rodriguez S, Butler FK. Causes of death in U.S. Special Operations Forces in the global war on terrorism: 2001–2004. Ann Surg. 2007;245(6):986–91.
- Perkins ZB, De'Ath HD, Aylwin C, Brohi K, Walsh M, Tai NR. Epidemiology and outcome of vascular trauma at a British Major Trauma Centre. Eur J Vasc Endovasc Surg. 2012;44(2): 203–9.
- Katzenell U, Ash N, Tapia AL, Campino GA, Glassberg E. Analysis of the causes of death of casualties in field military setting. Military Med. 2012;177(9):1065–8.
- Eastridge B, Mabry R, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, Butler F, Kotwal RS, Holcomb J, Wade C, Champion H, Lawnick M, Moores L, Blackborne L. Death on the battlefield (2001–2011): implications for the future of combat casulaty care. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S431–S7.
- Rhee P, Brown C, Martin M, Salim A, Plurad D, Green D, Chambers L, Demetriades D, Velmahos G, Alam H. QuikClot use in trauma for hemorrhage control: case series of 103 documented uses. J Trauma. 2008;64(4):1093–9.
- Wedmore I, McManus JG, Pusateri AE, Holcomb JB. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J Trauma. 2006;60(3):655–8.
- Brown MA, Daya MR, Worley JA. Experience with chitosan dressings in a civilian EMS system. J Emerg Med. 2009;37(1):1–7.
- Littlejohn LF, Devlin JJ, Kircher SS, Lueken R, Melia MR, Johnson AS. Comparison of Celox-A, ChitoFlex, WoundStat, and combat gauze hemostatic agents versus standard gauze dressing in control of hemorrhage in a swine model of penetrating trauma. Acad Emerg Med. 2011;18(4):340–50.
- Watters JM, Van PY, Hamilton GJ, Sambasivan C, Differding JA, Schreiber MA. Advanced hemostatic dressings are not superior to gauze for care under fire scenarios. J Trauma. 2011;70(6):1413–9.
- Kheirabadi B. Evaluation of topical hemostatic agents for combat wound treatment. US Army Med Dept J. 2011:25–37.
- Kheirabadi BS, Edens JW, Terrazas IB, Estep JS, Klemcke HG, Dubick MA, Holcomb JB. Comparison of new hemostatic granules/powders with currently deployed hemostatic products in a lethal model of extremity arterial hemorrhage in swine. J Trauma. 2009;66(2):316–26; discussion 27-8.
- McManus J, Hurtado T, Pusateri A, Knoop KJ. A case series describing thermal injury resulting from zeolite use for hemorrhage control in combat operations. Prehosp Emerg Care. 2007;11(1):67–71.
- Wright JK, Kalns J, Wolf EA, Traweek F, Schwarz S, Loeffler CK, Snyder W, Yantis LD, Jr., Eggers J. Thermal injury resulting from application of a granular mineral hemostatic agent. J Trauma. 2004;57(2):224–30.
- Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, Herrera J, Hunt B, Iribhogbe P, Izurieta M, Khamis H, Komolafe E, Marrero MA, Mejia-Mantilla J, Miranda J, Morales C, Olaomi O, Olldashi F, Perel P, Peto R, Ramana PV, Ravi RR, Yutthakasemsunt S. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32.
- Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, Gando S, Guyatt G, Hunt BJ, Morales C, Perel P, Prieto-Merino D, Woolley T. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011;377(9771):1096–101, 101 e1–2.
- Tactical Combat Casualty Care Committee. Tranexamic acid (TXA) in tactical combat casualty care: guideline revision recommendation. 2011. Available from: www.medicalsci.com/ files/tranexamic_acid_txa_in_tactical_combat_casualty_care.pdf.
- Kragh JF Jr, Murphy C, Dubick MA, Baer DG, Johnson J, Blackbourne LH. New tourniquet device concepts for battlefield hemorrhage control. US Army Medical Dept J. 2011:38– 48.
- Pannell D, Brisebois R, Talbot M, Trottier V, Clement J, Garraway N, McAlister V, Tien HC. Causes of death in Canadian Forces members deployed to Afghanistan and implications on tactical combat casualty care provision. J Trauma. 2011;71(5 Suppl 1):S401–7.
- North American Rescue. Junctional Emergency Treatment Tool. [Website] [cited 2013 April 18]; Available from: www. narescue.com/media/NAR/product-info-sheets/PIS-jett.pdf.
- Alam HB, Uy GB, Miller D, Koustova E, Hancock T, Inocencio R, Anderson D, Llorente O, Rhee P. Comparative analysis of hemostatic agents in a swine model of lethal groin injury. J Trauma. 2003;54(6):1077–82.
- Clay JG, Grayson JK, Zierold D. Comparative testing of new hemostatic agents in a swine model of extremity arterial and venous hemorrhage. Military Med. 2010;175(4): 280–4.
- Gustafson SB, Fulkerson P, Bildfell R, Aguilera L, Hazzard TM. Chitosan dressing provides hemostasis in swine femoral arterial injury model. Prehospital Emerg Care. 2007;11(2):172–8.
- Schwartz RB, Reynolds BZ, Shiver SA, Lerner EB, Greenfield EM, Solis RA, Kimpel NA, Coule PL, McManus JG. Comparison of two packable hemostatic gauze dressings in a porcine hemorrhage model. Prehospital Emerg Care. 2011;15(4):477–82.
- Alam HB, Chen Z, Jaskille A, Querol RI, Koustova E, Inocencio R, Conran R, Seufert A, Ariaban N, Toruno K, Rhee P. Application of a zeolite hemostatic agent achieves 100% survival in a lethal model of complex groin injury in swine. J Trauma. 2004;56(5):974–83.
- Arnaud F, Parreno-Sadalan D, Tomori T, Delima MG, Teranishi K, Carr W, McNamee G, McKeague A, Govindaraj K, Beadling C, Lutz C, Sharp T, Mog S, Burris D, McCarron R. Comparison of 10 hemostatic dressings in a groin transection model in swine. J Trauma. 2009;67(4):848–55.
- Arnaud F, Teranishi K, Okada T, Parreno-Sacdalan D, Hupalo D, McNamee G, Carr W, Burris D, McCarron R. Comparison of Combat Gauze and TraumaStat in two severe groin injury models. J Surg Res. 2011;169(1):92–8.
- Canadian Council on Animal Care. Guide for Use and Care Experimental Animals, 2nd ed. 1993.
- Sakaguchi M, Nishimura R, Sasaki N, Ishiguro T, Tamura H, Takeuchi A. Anesthesia induced in pigs by use of a combination of medetomidine, butorphanol, and ketamine and its reversal by administration of atipamezole. Am J Vet Res. 1996;57(4):529–34.
- Savage E, Forestier C, Withers N, Tien H, Pannell D. Tactical combat casualty care in the Canadian Forces: lessons learned from the Afghan war. Can J Surg. 2011;54(6):S118–23.
- Alam HB, Burris D, DaCorta JA, Rhee P. Hemorrhage control in the battlefield: role of new hemostatic agents. Military Med. 2005;170(1):63–9.
- Sauaia A, Moore FA, Moore EE, Haenel JB, Read RA, Lezotte DC. Early predictors of postinjury multiple organ failure. Arch Surg. 1994;129(1):39–45.
- Rotondo MF, Zonies DH. The damage control sequence and underlying logic. Surg Clin North Am. 1997;77(4):761–77.
- Rizoli SB, Scarpelini S, Callum J, Nascimento B, Mann KG, Pinto R, Jansen J, Tien HC. Clotting factor deficiency in early trauma-associated coagulopathy. J Trauma. 2011;71(5 Suppl 1):S427–34.
- Jansen JO, Thomas R, Loudon MA, Brooks A. Damage control resuscitation for patients with major trauma. BMJ. 2009; 338:b1778.
- Kragh JF Jr, Walters TJ, Baer DG, Fox CJ, Wade CE, Salinas J, Holcomb JB. Survival with emergency tourniquet use to stop bleeding in major limb trauma. Ann Surg. 2009;249(1):1–7.
- Kragh JF Jr, Littrel ML, Jones JA, Walters TJ, Baer DG, Wade CE, Holcomb JB. Battle casualty survival with emergency tourniquet use to stop limb bleeding. J Emerg Med. 2011;41(6):590–7.
- Acheson EM, Kheirabadi BS, Deguzman R, Dick EJ, Jr., Holcomb JB. Comparison of hemorrhage control agents applied to lethal extremity arterial hemorrhages in swine. J Trauma. 2005;59(4):865–74; discussion 74-5.