Abstract
Objective: Frameless image guided systems have traditionally been perceived as being less accurate than stereotactic frames, limiting their adoption for trajectory-based procedures such as deep brain stimulator placement which require submillimetric accuracy. However, some studies have suggested that high degrees of accuracy are attainable with optical localization systems. We evaluated the application accuracy of a skull-mounted trajectory guide coupled to an optical image-guided surgery system in a laboratory setting.
Materials and Methods: A plastic skull phantom was fitted with five fiducial markers rigidly attached via self-drilling bone screws. Varying MRI and CT imaging protocols were obtained at 25 different centers. A metal disc marked in 1-mm increments was placed at the expected target point. Following registration and alignment of the trajectory guide, radial and depth localization errors were measured. A total of 560 measurements were obtained and detailed statistical analyses were performed.
Results: Mean localization error was 1.25 mm with a 95% confidence interval of 2.7 mm and a 99.9% confidence interval of 4.0 mm. These values were significantly lower than those published for the two most widely used frame systems (p<0.001). Conclusions: Accuracy of image-guided localization using a rigid trajectory guide can meet or exceed that achievable with a stereotactic frame.
Introduction
Since its introduction in human neurosurgery by Spiegel and Wycis over 50 years ago Citation[1], the stereotactic frame has provided the standard targeting method for functional intracranial procedures such as pallidotomy, thalamotomy, and deep brain stimulator (DBS) placement. Until recently, stereotactic frames provided the only method for accurately localizing targets within the brain. With the development of image-guided neurosurgical systems over the past 10 years, alternative methods of delivering a surgical intervention to a well-defined target are now available. Theoretical advantages of a fully frameless functional surgery system include improved patient comfort; improved ability to perform intraoperative neurological evaluations; decoupling of imaging and surgery; real-time electrode tracking with integration of multiple information sources; and the potential for increased accuracy Citation[2], Citation[3]. However, the accuracy of these systems in routine use has generally been felt to be insufficient for the performance of highly precise procedures such as DBS. This perception has been based on two widely-held beliefs: 1) that stereotactic frames can provide consistent localization accuracies of less than 1 mm; and 2) that frameless image-guided localization is inherently less accurate than that achievable with a frame.
As elucidated by Maciunas et al. in their landmark study of stereotactic frames Citation[4], the application accuracy of a stereotactic system represents the accuracy which a surgeon can expect in real-world use. This measure thus incorporates not only mechanical precision, but also errors inherent in imaging, registration, and target localization. While the mechanical accuracy of a stereotactic frame may indeed be submillimetric, the application accuracy is much less than this idealized figure Citation[4]. Several laboratory studies of image-guided surgical systems demonstrated localization accuracies similar to those achievable with a stereotactic frame Citation[5–8]. Preliminary studies have shown the feasibility of using a surgical navigation system in conjunction with an image-guided microdrive to perform functional neurosurgical procedures Citation[3] with acceptable accuracy. The NeXframe (Image Guided Neurologics, Melbourne, FL) is a skull-mounted platform developed specifically for accurate targeting in frameless trajectory-based procedures. Rigorous laboratory testing of the device was undertaken to evaluate whether it would provide sufficient accuracy for clinical use as an equivalent to a stereotactic frame.
Materials and methods
Testing was performed in a plastic skull phantom with an internal target oriented perpendicular to the expected trajectory of probe insertion (). The target was a metal disc of 5 mm diameter, marked in 1-mm increments to allow measurement of localization error to within approximately 0.25 mm in a radial direction, and designed to fit into the collar of a standard skull fiducial in a reproducible fashion (). Five implantable bone fiducials (Stryker Leibinger, Portage, MI) were screwed into the phantom skull and spherical markers were snapped into place. The markers were hollow plastic spheres filled with a proprietary copper sulfate solution. A sixth fiducial base was screwed into a platform within the skull phantom to serve as a target, and a spherical marker placed. The location of the target within the phantom was intended to represent the approximate position of usual stereotactic targets for deep brain stimulation or lesioning.
Figure 1. The plastic skull phantom used in this study attached to a Leksell frame for stability. The first generation NeXframe device is mounted over the right-sided entry point and the stainless steel mandrel localizes the target. A cranial reference arc is indicated by the asterisk(*).

The phantom was then filled with water and a T1-weighted MRI scan was acquired. Imaging protocols varied between institutions, representing the routine studies used for stereotactic procedures at each site. Nine different models of MRI scanner were used at 25 sites, with the four major equipment manufacturers (Siemens, GE, Picker and Phillips) represented. No attempt was made to standardize imaging protocols, as the objective of the study was to produce a composite figure of application accuracy which would be broadly applicable across platforms and between sites. Slice thicknesses varied between 1.0 and 2.0 mm, with some datasets acquired as slice stacks and others acquired as volumes. A CT scan with slice thicknesses varying from 1.0 to 2.0 mm was then obtained, depending on the routine stereotactic imaging protocol at each institution. Seven different models of CT scanner were used, again representing all four major equipment manufacturers. Both image datasets were loaded into the StealthStation surgical workstation and image fusion was performed using the FrameLink planning software (Medtronic SNT, Louisville, CO). Planning was then carried out as for a standard functional stereotactic procedure, using the center of the marker sphere within the phantom as the target. The marker sphere was then replaced with the target disc.
The NeXframe trajectory guide was then anchored in place with three bone screws over a pre-drilled burrhole which served as the entry point for all trials. A reference frame was then attached to the phantom by one of several methods. In the earliest trials, a stereotactic frame was applied to the phantom skull and a cranial reference arc was attached to the frame. In later trials, a small, active 4-emitter frame was attached directly to the skull with a bone anchoring system. In the most recent trials, a reference arc equipped with passive reflectors was attached directly to the NeXframe tower with mounting screws designed for this purpose.
The five spherical fiducial markers were replaced by registration divots, and a registration probe was used to touch each fiducial in turn. Registration error was recorded. A biopsy guide tube equipped with four light-emitting diodes (LEDs) was then used to align the trajectory of the NeXframe device with the planned target-entry projection, and the device was locked in position. A stainless steel mandrel was inserted to the target depth as predicted by the Stealth Cranial software. Measurements of radial error (to the nearest 0.25 mm) and depth error were then taken (). Depth error was measured with a stainless steel ruler marked in 0.5-mm increments, again allowing for a 0.25-mm error estimate. The locking screws on the NeXframe guide were then loosened, the device was rotated through both degrees of freedom, and aiming was repeated, again locking the device in place prior to introducing the mandrel. This process of re-aiming and error measurement was carried out 5 times, following which a re-registration was performed. Five trials of localization were performed for each of three registrations, resulting in a total of 15 measurements of localization error for each trial. A total of 560 accuracy measurements were performed.
Statistical analysis
For each measurement, the root-mean-square localization error was calculated using the formula (radial error2+depth error2)1/2. As noted by Maciunas et al. Citation[4], these errors cannot be absolutely normally distributed because negative errors cannot occur. However, the data are distributed in a quasinormal fashion, which is reliably predictable when analyzed in terms of a Gaussian distribution (). Data from all centers were combined and analyzed for mean, median, symmetry, skew, and kurtosis, and were studied with box plots and KS testing as described by Maciunas et al. Citation[4].
Figure 4. Histogram plot of localization errors in 560 trials, showing quasinormal distribution around the mean of 1.25 mm.
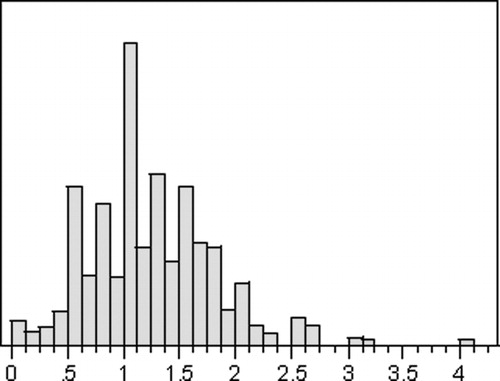
Localization error was reported in terms of the mean, standard deviation, minimum, and maximum values. Standard error of the mean (SEM) was calculated as SEM=SD/(n)1/2, where SD=standard deviation and n=number of sample observations (560). The 99% confidence interval for the accuracy of the sample means was expressed as (Mean−3 SEM to Mean+3 SEM). Ninety-five and 99.9% confidence intervals were determined both by a right-tailed test and by quantile analysis. Given the relatively small n as compared to the study by Maciunas et al., the quantile analysis produced larger intervals and was felt to more accurately represent the true confidence intervals of the data. The results are summarized in .
Table I. The application accuracy of two stereotactic frame systems as compared to the frameless device.
Each cell in column 3 of was compared with the corresponding cell in columns 1 and 2 using a two-sample (unpaired) t-test. In each case there was a statistically significant difference between the two measurements (p<0.001).
Results
Localization errors ranged from 0 mm to 4.0 mm, with a mean error and standard deviation of 1.25±0.57 mm. The 99% confidence interval for the mean was 1.19 to 1.31 mm. Ninety-five and 99.9% confidence intervals for the data were 2.7 and 4.0 mm, respectively. As discussed above, the application of a right-tailed test to the data produced confidence intervals which were felt to be artificially decreased compared to those obtained by using a quantile analysis, which would tend to overestimate the accuracy of the device. We thus chose to use the quantile analysis as being more representative of the true 95 and 99.9% confidence intervals. presents a summary of these results, with comparison to the measurements of Maciunas et al. for the Radionics Cosman-Roberts-Wells (CRW) frame (Radionics, Burlington, MA) and the Leksell Model G frame (Elekta Instruments, Norcross, GA).
The differences between the localization errors reported for the NeXframe and each of two widely used stereotactic frame systems (CRW and Leksell) were statistically significant (p<0.001). Thus, for the specific conditions described in this study, the localization accuracy of the frameless device was superior to either frame.
Discussion
Despite the widespread adoption of frameless image-guided systems for open cranial and spinal procedures, frame-based systems have still been generally preferred for cranial procedures which require a fixed trajectory, such as brain biopsy, deep brain stimulator placement, or lesioning of the deep nuclei. Accuracy in these cases is particularly important, due to the fact that small deviations of trajectory can lead to large localization errors at the target depth. In an analysis of the application accuracy of stereotactic frames, Maciunas et al. showed that the 95% confidence interval for localization accuracy of the two most popular frame systems under optimal imaging conditions (CT slices 1 mm thick) lies somewhere between 3.4 and 3.6 mm Citation[4]. By comparison, the 95% confidence interval for localization accuracy of the infrared-based frameless system used in this study was 2.7 mm, agreeing closely with the 2.75 mm reported previously Citation[5]. Several laboratory studies have examined a wide variety of frameless localization systems, reporting mean localization accuracies ranging from 0.9 mm Citation[6] to 2.61 mm Citation[8]. In the laboratory setting, the localization accuracy of framed and frameless systems appears to be nearly equivalent (). Frameless systems have demonstrated their clinical utility for guiding needle biopsies of deep-seated lesions in several published reports Citation[6],Citation[9–11]. A preliminary study of frameless localization for functional neurosurgical procedures demonstrated the feasibility of this approach in both the laboratory and clinical settings Citation[3].
Table II. A comparison of laboratory studies investigating the mean localization accuracy of framed and frameless stereotactic systems
To achieve optimal accuracy with either framed or frameless systems, it is important to maintain a fixed relationship between the fiducial markers and the target of interest. Frame-based systems decouple the N-bar fiducial system from the skull, leading to potential localization errors due to the shift of the fiducial system during imaging and repositioning Citation[12]. This limitation applies even more strongly to frameless procedures using fiducials affixed to the scalp, which is a mobile structure. To date, all of the published accuracy studies and the majority of the laboratory studies of frameless systems have used adhesive “doughnut” markers or anatomical fiducials Citation[5–8],Citation[10], Citation[11], Citation[13–16] (). There are several factors which contribute to inaccuracy when using this type of fiducial marker, including not only mobility of the fiducial with reference to the structure of interest, but also difficulty in precisely localizing the center of the fiducial in phantom studies and difficulty in finding the exact registration point on the imaging study. Optimal fiducials would be rigidly fixed to the skull and would have a known geometry, so that the precise center could be accurately and reproducibly localized. In fact, localization accuracies of 0.7 mm have been shown in clinical use with this type of system Citation[2], which exceeds the accuracy of a stereotactic frame. Given these factors, the current system was designed from the outset to be used with implantable bone fiducials in order to maximize theoretical accuracy.
Frameless functional surgery presents a number of challenges when compared to traditional frame-based functional surgery or to other image-guided neurosurgical procedures. Extra steps are required for the registration of patient position to imaging studies in the operating room. Fiducial placement is slightly more time-consuming than frame placement when performed by a surgeon experienced in frame placement. However, there are some possible advantages to a frameless approach, including increased patient comfort and mobility, as well as improvements in targeting accuracy when compared to a stereotactic frame. Real-time electrode tracking greatly improves the surgeon's ability to integrate multiple information sources into the OR display, and could potentially improve “situational awareness” and targeting accuracy Citation[17]. By placing the fiducials one or more days prior to surgery, imaging and planning can be decoupled from the act of surgery, allowing more efficient use of operating room time.
There is a perception that frameless localization systems lack the required accuracy for functional neurosurgery. However, as this study demonstrates, the application accuracy of the current system closely matches or exceeds that reported for stereotactic frames. Further studies are currently underway to evaluate whether this accuracy will be sustained in the clinical setting, leading to the possibility of fully frameless functional procedures.
Financial disclosures
Dr. Holloway has received research support and honoraria from Image Guided Neurologics. Dr. Henderson and Dr. Rosenow currently serve as consultants for Image Guided Neurologics.
References
- Spiegel E A, Wycis H T, Marks M, Lee A J. Stereotactic apparatus for operations on the human brain. Science 1947; 106: 349–50
- Maciunas R J, Fitzpatrick J M, Galloway R L, Allen G S. Beyond stereotaxy: extreme levels of application accuracy are provided by implantable fiducial markers for interactive image guided neurosurgery. Interactive Image Guided Neurosurgery, R J Maciunas. AANS Publications, Park Ridge, IL 1993; 259–70
- Henderson J M. Frameless localization for functional neurosurgical procedures: A preliminary accuracy study. Stereotactic & Functional Neurosurgery 2004; 82: 135–41
- Maciunas R J, Galloway R L, Latimer J W. The application accuracy of stereotactic frames. Neurosurgery 1994; 35: 682–94
- Steinmeier R, Rachinger J, Kaus M, Ganslandt O, Huk W, Fahlbusch R. Factors influencing the application accuracy of neuronavigation systems. Stereotactic & Functional Neurosurgery 2000; 74: 188–202
- Dorward N L, Alberti O, Palmer J D, Kitchen N D, Thomas D GT. Accuracy of true frameless stereotaxy: in vivo measurement and laboratory phantom studies. J Neurosurg 1999; 90: 160–8
- Helm P A, Eckel T S. Accuracy of registration methods in frameless stereotaxis. Comput Aided Surg 1998; 3: 51–6
- Bernardete E A, Leonard M A, Weiner H L. Comparison of frameless stereotactic systems: accuracy, precision, and applications. Neurosurgery 2001; 49: 1409–16
- Germano I M, Queenan J V. Clinical experience with intracranial brain needle biopsy using frameless surgical navigation. Comput Aided Surg 1998; 3: 33–9
- Stechison M T. A digitized biopsy needle for frameless stereotactic biopsies with the StealthStation. Neurosurgery 2000; 46: 239–41
- Paleologos T S, Dorward N L, Wadley J P, Thomas D GT. Clinical validation of true frameless stereotactic biopsy: analysis of the first 125 consecutive cases. Neurosurgery 2001; 49: 830–7
- Rohlfing T, Maurer C R, Dean D, Maciunas R J. Effect of changing patient position from supine to prone on the accuracy of a Brown-Roberts-Wells stereotactic head frame system. Neurosurgery 2003; 52: 610–8
- Golfinos J G, Fitzpatrick B C, Smith L R, Spetzler R F. Clinical use of a frameless stereotactic arm: results of 325 cases. J Neurosurg 1995; 83: 197–205
- Sipos E P, Tebo S A, Zinreich S J, Long D M, Brem H. In vivo accuracy testing and clinical experience with the ISG viewing wand. Neurosurgery 1996; 39: 194–204
- Germano I M, Villalobos H, Silvers A, Post K D. Clinical use of the optical digitizer for intracranial neuronavigation. Neurosurgery 1999; 45: 261–70
- Kitchen N D, Lemieux L, Thomas D GT. Accuracy in frame-based and frameless stereotaxy. Stereotactic & Functional Neurosurgery 1993; 61: 195–206
- Henderson J M. The role of computer-assisted image-guided techniques. Seminars in Neurosurgery 2001; 12: 175–81

