Abstract
Objective: Otologic surgery is undertaken to treat ailments of the ear, including persistent infections, hearing loss, vertigo, and cancer. Typically performed on otherwise-healthy patients in outpatient facilities, the application of image-guided surgery (IGS) has been limited because accurate (<1 mm), non-invasive fiducial systems for otologic surgery have not been available. We now present such a fiducial system.
Methods: A dental bite-block was fitted with a custom-designed rigid frame with 7 fiducial markers surrounding each external ear. The bones containing the ear (i.e., the temporal bones) of 3 cadaveric skulls were removed and replaced with discs containing 13 surgical targets arranged in a cross-hair pattern about the centroid of each ear. The surgical targets (26/skull) and fiducial markers (14/skull) were identified both within CT scans using a published algorithm and in physical space using an infrared optical tracking system. Fiducial registration error (FRE), fiducial localization error (FLE), and target registration error (TRE) were calculated.
Results: For all trials, root mean square FRE=0.66, FLE=0.72, and TRE=0.77mm. The mean TRE for n=234 independent targets was 0.73 with a standard deviation of 0.25mm.
Conclusions: Using a novel, non-invasive fiducial system (the EarMark™), submillimetric accuracy was repeatably achieved. This system will facilitate image-guided otologic surgery.
Introduction
During the past decade Image-Guided Surgery (IGS) has altered the surgical landscape. In neurosurgery, it has become standard for use in tumor biopsy and excision where rigid frames (i.e. N-frames) or bone-implanted markers are employed in linking preoperative radiographic studies to intraoperative anatomy. Stereotactic frames are invasive and cumbersome, but are tolerated by patients with life-threatening diseases such as malignant brain tumors. In such situations IGS systems have been shown to decrease intraoperative time Citation[1] and allow more complete resection of diseased tissue with less collateral damage to healthy tissue Citation[2].
To facilitate use of IGS in more routine surgical interventions, research has focused on minimally invasive fiducial systems. A major improvement over stereotactic frames has been the use of bone-implanted fiducial markers. The only bone-implanted fiducial currently approved by the FDA, the Acustar® marker system (Z-Kat Inc., Hollywood, FL), achieves accuracy comparable to, or better than, that of rigid N-bar frames Citation[3], Citation[4]. Less invasive fiducial systems, including skin-affixed fiducial markers and surface-matching protocols (e.g., laser contouring), are less accurate. Skin-affixed markers produce accuracies in the range of 1.5 mm, while laser skin-contouring produces accuracies in the range of 2.5 mm Citation[5], Citation[6]. While improvements continue with skin-affixed and surface-matching fiducial strategies, bone-affixed fiducial markers achieve superior accuracy.
Otologic surgery is undertaken to treat ailments of the ear, including persistent infections, hearing loss, vertigo and cancer. It is unique in that the target organ is embedded in a rigid framework – the temporal bone. The goal of the surgical intervention is to remove diseased tissue and bone while preserving bone containing vital structures (the inner ear, neural tissue, etc.). This goal often necessitates surgical accuracy of better than 1 mm. The majority of otologic surgical procedures occur on an outpatient basis, with patients being both admitted and discharged from the hospital on the same day. If IGS is to find utility in this large and vibrant healthcare market, non-invasive fiducial systems with high levels of accuracy must be made available.
Our objective was to find a solution to the seemingly paradoxical design criteria of (i) submillimetric accuracy, reliably achieved only with bone-implanted frames or markers, and (ii) non-invasive application facilitating outpatient, otologic procedures. The solution lay in the only normally exposed part of the skull – the teeth. The maxilla, or upper jaw, is one of the most stable bones in the body. External devices can be easily anchored to it via dental bite-blocks. At least three groups have capitalized on this approach: The first used a dental cast of the maxilla and upper dentition held in place by a vacuum system Citation[7]; the second devised a maxillary bite-plate system for frameless stereotactic radiosurgery Citation[8]; while the third developed the locking dental stent (LADS). The LADS is a three-component unit similar to an athletic mouthguard. It consists of a central piece which engages the biting surfaces of the teeth, as well as right and left buccal pieces which engage the lateral surfaces of the teeth. The three pieces are attached together with screws that lock the components around the crowns of the teeth, holding the mouthpiece reliably in place while allowing it to be removed and replaced in the same position and orientation Citation[9].
Building on the LADS dental bite-block, we constructed a lightweight yet strong frame to hold fiducial markers in a pattern surrounding both external ears. This frame (the EarMark™) is worn when obtaining preoperative radiographic studies by CT or MRI. Intraoperatively, the patient wears the LADS with the fiducial frame replaced by an infrared (IR) emitter, which is significantly smaller than the frame. After registration, otologic surgery can be undertaken. Herein we describe the EarMark™ system and its bench-top validation, including detailed analysis of fiducial registration error (FRE), fiducial localization error (FLE), and target registration error (TRE).
Materials and methods
The EarMark™ system () has a frame constructed of carbon-fiber tubing of inner diameter 1.0 inches, outer diameter 1.12 inches, and wall thickness of 0.060 inches (Maclean Quality Composites, West Jordan, UT). Carbon-fiber tubing was chosen because it provides a superior strength-to-weight ratio and is compatible with both MRI and CT. Total weight of the system is 8 ounces (<230 g). Affixed to the midpoint of the front bar is a custom-designed coupling system with a recessed slot to accommodate the LADS, allowing accurate coupling of the LADS to the EarMark™.
Figure 1. The EarMark™ system as arranged for a patient's radiographic studies (i.e., CT scanning). At top left is a side view, with an overhead view at top right. The EarMark™ is composed of carbon fiber. It is coupled to the LADS (which has the appearance of a mouthguard) via a customized block epoxied to the EarMark™, allowing a screw-fit to the LADS. At bottom is the numbering scheme for the fiducial markers. These numbers are used in data reporting in , , and .
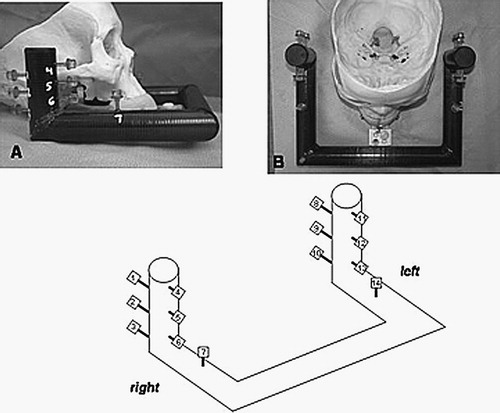
Fiducial markers (Acustar, Z-Kat, Inc., Hollywood, FL) were arranged on the EarMark™ based on previous studies by the senior author showing that accuracy is improved by (1) increasing the number of fiducial markers, (2) widely spacing the markers, and (3) arranging them such that their centroid approximates the target of interest Citation[10]. Building on this theory, the EarMark™ was designed to satisfy requirement (1) by simply including a large number of fiducial markers. Preliminary experiments showed that submillimetric accuracy was maintained when a critical number of markers (n=6) per side were used Citation[11]. To provide an increased margin of accuracy, 7 fiducial markers were installed per side. To satisfy requirement (2), the fiducial markers were positioned such that wide spacing was achieved in all three dimensions. This necessitated placing two fiducial markers (markers 7 and 14) on the horizontal portion of the EarMark™. As for requirement (3), the geometric arrangement, the markers were positioned just lateral to each side of the temporal bone so that the centroid of the markers approximated the center of the surgical field.
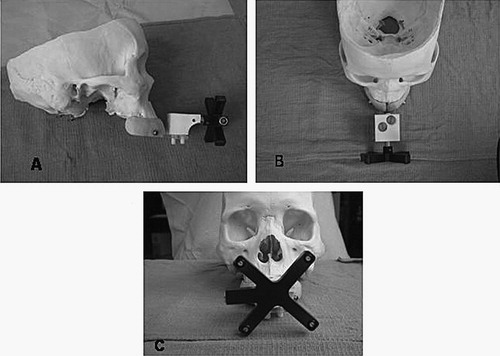
The set-up shown in is the arrangement that is worn for radiographic studies (CT scans in the current study). Since most otologic procedures are done on an outpatient basis with a low incidence of complications, the EarMark™ was designed to be both easy to use and non-interfering during surgery. Thus, a separate set-up, shown in , was designed for intraoperative use. This consists of the LADS attached to an IR emitter. To map the position of the IR emitter to the fiducial markers both the IR emitter and EarMark are simultaneously attached to the LADS. Once this transformation is determined, the LADS with attached IR emitter () is sufficient for accurate registration.
Figure 2. The EarMark™ system as arranged for operative use. Panel A shows a side view, Panel B an overhead view, and Panel C a frontal view. The mouthpiece-like LADS is seen in blue with a Plexiglas extender jutting out anteriorly. The infrared (IR) emitter is the black X-shaped device. This is connected to the LADS via the customized adapter (in white) which allows positioning of the IR emitter in an identical fashion both with and without the EarMark™.
Using this system (LADS+EarMark™), accuracy within the region of the temporal bone was investigated. To accomplish this, three separate skulls were analyzed as follows. First, each skull was custom-fitted with a LADS as previously described Citation[9]. Next, the temporal bones were removed to allow rigid fixation of a customized target disc system. This cross-shaped system, shown in , allowed placement of surgical targets centered about the center of the temporal bone and radiating out in orthogonal directions. A disc was placed in a horizontal position on one side of the skull, and another disc placed in a vertical position on the contralateral side. Thirteen surgical target markers were placed on each disc. Thus, each skull had 14 fiducial markers attached to the EarMark™ and 26 surgical targets radiating outward from the centroids of the temporal bones.
Figure 3. The experimental set-up for determining target registration error within the region of the temporal bone. Illustrated is one of the three skulls which has had the temporal bones removed and replaced with 2 discs, each holding 13 surgical targets arranged in a cross-hair pattern over the approximate centroid of the temporal bone. The left photograph shows the vertical disc while the right photograph shows the horizontal disc. The respective identification systems are shown in the schematics beneath the photographs. These identifiers are used in data reporting in , and .
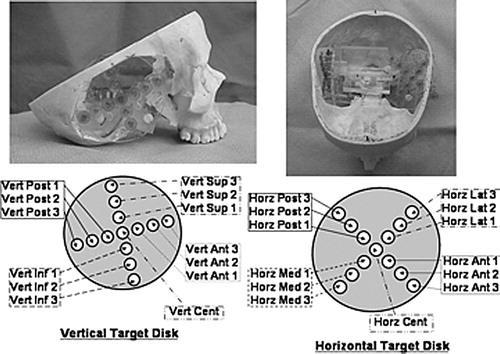
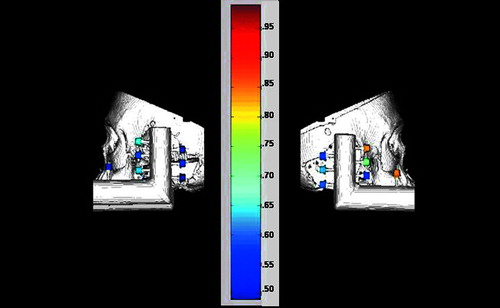
Figure 4. Graphical representation of Target Registration Errors (TREs). Shown are 3D reconstructions of the skull and target system from the CT scans. The surgical targets are colored according to error (blue=least error, red=most error; a numerical scale corresponding to colors is shown at right). The left panel presents the horizontal disc best, while the right panel presents the vertical disc best.
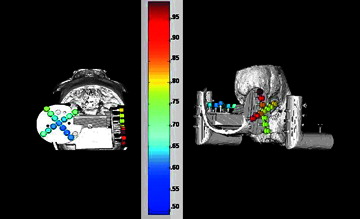
Figure 5. Graphical representation of the Fiducial Registration Errors (FREs). Shown are 3D reconstructions of the skull and fiducial frame from the CT scans. The fiducials are colored according to error (blue=least error, red=most error; a numerical scale corresponding to colors is shown at right). The left panel presents the left side (fiducials #8–14), while the right panel presents the right side (fiducials #1–7) (see for fiducial numbering scheme).
Each skull, complete with LADS, EarMark™ and surgical targets, was then CT-scanned using clinically applicable temporal bone algorithms (slice thickness=0.5 mm). Three CT scans were obtained for each skull with the skull repositioned between scans. The digital file from each CT scan was then analyzed to detect the centroid of each marker (both the fiducial markers attached to the EarMark™ frame as well as the markers serving as surgical targets). This was accomplished using a previously described computer program Citation[12]. Next, each skull was transported back to our mock operating room laboratory where the LADS were removed and reapplied for each skull. The markers' locations in physical space (both the fiducials and the targets) were measured using a customized 3D localization system based on a commercially available infrared tracking system (Polaris R, Northern Digital, Inc., Waterloo, Ontario, Canada) and optical triangulation driven by an image analysis, and a display system (Voyager, Z-Kat, Inc.) running on a 1 GHz Intel PC system (Dell, Inc.) under Linux (Red Hat, Inc.). Three such physical space acquisitions were accomplished for each skull.
Rigid registration between physical space (the mock operating room) and radiographic space (the CT scan) was performed using the 14 fiducial markers (7 on each side) on the EarMark™ system with a closed-form algorithm based on the singular-value decomposition of the cross-covariance matrix of the physical and radiographic marker positions to minimize Fiducial Registration Error (FRE) Citation[13]. Thus, 9 independent FREs were calculated: 3 skulls each with 3 separate radiographic and physical space registrations. As reported below, FRE is, by definition, the root mean square distance between corresponding fiducials in physical and radiographic space. From FRE, Fiducial Localization Error (FLE), defined as the error in locating the fiducial markers, was calculated as previously described Citation[13].
To determine the Target Registration Error (TRE), defined as the root mean square distance between corresponding points other than fiducials in physical space versus radiographic space, a method used by multiple groups to retrospectively compare registration techniques was employed Citation[14]. In this technique, a “gold standard” registration is established using the target markers as fiducials. Applied to the current project, the 26 surgical targets located on the discs were used as fiducials in registering physical space to radiographic space. A comparison of the two registrations (fiducials as fiducials and targets as fiducials) was then made to determine the combined error associated with relative transformation. Each resulting transformation was applied to each surgical target marker in physical space. TRE could then be determined as the disparity between the transformed position and the measured position. These values for TRE are reported below.
Results
Data is presented in tabular form as follows: – TRE for Skull 1; – FRE for Skull 1; – TRE for Skull 2; – FRE for Skull 2; – TRE for Skull 3; – FRE for Skull 3. Corresponding locations of targets are shown in and for fiducials in .
Table I. Target registration errors for Skull 1.
Table II. Fiducial registration errors for Skull 1.
Table III. Target registration errors for Skull 2.
Table IV. Fiducial registration errors for Skull 2.
Table V. Target registration errors for Skull 3.
Table VI. Fiducial registration errors for Skull 3.
TRE: As previously reported, Citation[15], a total of 234 independent calculations of TRE were carried out with a mean value of 0.73 mm, a standard deviation of 0.25 mm, and a root mean square of 0.77 mm. Minimum TRE was 0.16 mm and maximum was 1.66 mm. Of 234 TREs, 195 (83%) were less than 1 mm. Of the 39 errors greater than or equal to 1 mm, 11 occurred with Skull 1, 3 with Skull 2, and 25 with Skull 3. The eleven outliers noted with Skull 1 had a maximum value of 1.2 and all occurred during Trial 3. The three outliers noted with Skull 2 occurred randomly and had values of 1.00, 1.03 and 1.20. The vast majority of outliers (25) occurred with Skull 3 and were all noted within the horizontal disc.
FRE: A total of 124 independent calculations of FRE were performed. One fiducial marker was outside the CT scan limits for Skull 3, Trials 2 and 3. Overall analysis showed a mean of 0.60 mm, a standard deviation of 0.28 mm, and a root mean square of 0.66 mm. The largest FRE was 1.41 mm and the smallest 0.12 mm. Of 124 FREs, 113 (91%) were less than 1 mm. Of the 11 errors greater than or equal to 1 mm, none occurred with Skull 1, three occurred with Skull 2, and eight occurred with Skull 3. The vast majority of outliers (6) occurred with Skull 3, Trial 1.
FLE: FLE was calculated to be 0.55 mm for Skull 1, 0.66 mm for Skull 2, and 0.89 mm for Skull 3. Root mean square for all trials was 0.72 mm.
Discussion
At present, no commercially available IGS systems are geared toward otologic surgery. There are isolated case reports of their use in patients with unusual anatomy. Sargent and Bucholz Citation[16] utilized a modified neurosurgical unit to approach the inner ear from above via a middle cranial fossa approach. Raine et al. Citation[17] reported the use of an IGS system for a customized cochlear implant placement in a patient with an abnormally ossified inner ear. Caversaccio et al. Citation[18] recently reported on the use of IGS systems in repairing aural atresia, a condition in which the ear canal does not properly develop. While current IGS systems have the flexibility to allow their use in unusual cases, our intent was to develop a system for use in routine otologic cases, as the incidence of ear disease necessitating surgery (excluding myringotomy with tube placement [i.e., ear tubes] used primarily to treat children's ear infections) is approximately 10–100/100,000 Citation[19], Citation[20].
Hypothetically, the use of IGS in otologic surgery has been limited by (1) the need for submillimetric accuracy via a system which is (2) non-invasive and (3) easy to use. Submillimetric accuracy is necessary as multiple vital structures are embedded within the temporal bone and are in close proximity during otologic surgery. These include the facial nerve (injury results in paralysis of the ipsilateral face), the inner ear (injury results in permanent hearing loss and vertigo), the floor of the cranial vault (injury results in leakage of cerebrospinal fluid), and the internal jugular vein and carotid artery (injury results in blood loss which may be life threatening). We have overcome these limitations through the novel EarMark™ fiducial system, which non-invasively and easily attaches to a patient's dentition and achieves accuracy necessary to avoid damage to collateral tissue.
A potential limitation of the system is the need for adequate dentition. For the LADS, reliable fixation is achieved with a minimum of two stable maxillary teeth on each side of the midline. An alternate solution is the use of a dental splint (i.e., a denture plate) attached to the roof of the mouth with adhesive during radiographic imaging and then screwed into the roof of the mouth during the operation. Another group has overcome this problem with a vacuum-affixed mouthpiece which is not dependent upon dentition Citation[7]. This device is complicated, however, by the need for an additional air tube coming out of the patient's mouth which can be confused with the life-sustaining endotracheal tube used to ventilate patients during surgery.
Regarding accuracy, overall RMS values for TRE, FRE, and FLE are 0.77 mm, 0.66 mm, and 0.72 mm, respectively. Reviewing our data, we noted that the largest errors occurred when analyzing the horizontal targets in the third skull. This raises questions as to whether the horizontal disc moved slightly between CT scanning and mock operating room analysis. Regardless, we included this data in our analysis and still achieved submillimetric TRE.
Comparison of the current results to other systems is difficult because reported error analysis varies greatly. The standard analysis pioneered by the senior author Citation[3], Citation[4], Citation[10],Citation[12–14] with reporting of FRE, FLE, and TRE is not universally followed. This is especially true for commercially available, FDA-approved, clinical IGS systems for which advertisements frequently list numeric values for errors without specifying which error (target registration, fiducial registration, or fiducial localization) or which method (i.e., singular decomposition) was used. Of interest to the clinician is the target registration error – the difference between the actual location of a surgical target and the location identified using image guidance. Data extracted from peer-reviewed publications provides a best estimate of the TRE for the following IGS systems: LandmarX™ (Xomed, Inc., Jacksonville, FL): 1.69±0.38 mm Citation[20]; BrainLAB (BrainLAB, Heimstetten, Germany): 1.31±0.87 for skin-affixed markers and 2.77±1.64 mm for laser contouring Citation[22]; and InstaTrak (GE Medical Systems, Lawrence, MA): 2.28±0.91 mm Citation[23]. The EarMark™ compares favorably with these systems.
The EarMark™ fiducial marker system, currently in clinical trials on patients undergoing elective ear surgery, has widespread application within the field of otology/neurotology. Analogous to IGS systems in neurosurgery, orthopaedic surgery, and laparoscopic surgery, we hypothesize that otologic IGS will be cost effective, expeditiously allowing more extensive removal of diseased tissue with minimal collateral damage. With this platform, the concept of robotic otologic surgery is also feasible and ongoing research in our laboratory is directed at reducing this idea to practice.
Conclusion
The EarMark™ system is a novel, non-invasive fiducial frame which directly couples to a patient's maxillary dentition. Ex-vivo analysis presented in this paper has shown submillimetric target registration error within the surgical field of interest, i.e., the temporal bone.
Acknowledgments
This work was supported by Vanderbilt University Medical Center (Discovery Grant, PI – RFL) and the National Institute of Biomedical Imaging and Bioengineering (R21 EB02886–01, PI – RFL).
References
- Weinberg J S, Lang F F, Sawaya R. Surgical management of brain metastases. Curr Oncol Rep 2001; 3(6)476–83
- Wisoff J H, Boyett J M, Berger M S, Brant C, Li H, Yates A J, McGuire-Cullern P, Turski P A, Sutton L N, Allen J C, Packer R J, Finlay J L. Current neurosurgical management and the impact of the extent of resection in the treatment of malignant gliomas of childhood: a report of the Children's Cancer Group trial no. CCG-945. J Neurosurg 1998; 89(1)52–9
- Maurer C R, Jr, Fitzpatrick J M, Galloway R L, Jr, Wang M Y, Maciunas R J, Allen G S (1995) The accuracy of image-guided neurosurgery using implantable fiducial markers. Proceedings of the International Symposium on Computer and Communication Systems for Image Guided Diagnostics and Therapy (CAR '95), Berlin, June, 1995, H U Lemke, K Inamura, C C Jaffe, M W Vannier. Springer, Berlin, 1197–1202, Computer Assisted Radiology
- Maurer C R, Jr, Fitzpatrick J M, Wang M Y, Galloway R L, Jr, Maciunas R J, Allen G S. Registration of head volume images using implantable fiducial markers. IEEE Trans Med Imaging 1997; 16(4)447–62
- Raabe A, Krishnan R, Wolff R, Hermann E, Zimmermann M, Seifert V. Laser surface scanning for patient registration in intracranial image-guided surgery. Neurosurgery 2002; 50: 797–803
- Schlaier J, Warnat J, Brawanski A. Registration accuracy and practicability of laser-directed surface matching. Comput Aided Surg 2002; 7: 284–90
- Bale R J, Burtscher J, Eisner W, et al. Computer-assisted neurosurgery by using a noninvasive vacuum-affixed dental cast that acts as a reference base: another step toward a unified approach in the treatment of brain tumors. Neurosurg 2000; 93: 208–13
- Meeks S L, Bova F J, Wagner T H, et al. Image localization for frameless stereotactic radiotherapy. Int J Radiat Oncol Biol Phys 2000; 46: 1291–9
- Fenlon M R, Jusczyzck A S, Edwards P J, King A P. Locking acrylic resin dental stent for image guided surgery. J Prosthet Dent 2000; 83: 482–5
- West J B, Fitzpatrick J M, Toms S, et al. Fiducial point placement and the accuracy of point-based, rigid-body registration. Neurosurg 2001; 48: 810–17
- Labadie R F, Fenlon M, Devikalp H, . Image-guided otologic surgery. Computer Assisted Radiology and Surgery. Proceedings of the 17th International Congress and Exhibition (CARS 2003), London, H U Lemke, M W Vannier, K Inamura, A G Farman, K Doi, J HC Reiber, et al. Elsevier, Amsterdam, June 2003, 627–32
- Wang M Y, Maurer C R, Jr, Fitzpatrick J M, Maciunas R J. An automatic technique for finding and localizing externally attached markers in CT and MR volume images of the head. IEEE Trans Biomed Eng 1996;; 43: 627–37
- Fitzpatrick J M, West J M, Maurer C R, Jr. Predicting error in rigid-body, point-based registration. IEEE Trans Med Imaging 1998; 17: 694–702
- West J, Fitzpatrick J M, Wang M Y, et al. Comparison and evaluation of retrospective intermodality image registration techniques. J Comput Assist Tomogr 1997; 21: 554–66
- Labadie R F, Shah R J, Harris S S, et al. In vitro assessment of image-guided otologic surgery: Submillimeter accuracy within the region of the temporal bone. Otolaryngol Head Neck Surg 2005; 132: 435-42
- Sargent E W, Bucholz R D. Middle cranial fossa surgery with image-guided instrumentation. Otolaryngol Head Neck Surg 1997; 117: 131–4
- Raine C H, Strachan D, Gopichandran T. How we do it: Using a surgical navigation system in the management of the ossified cochlea. Cochlear Implants International 2003; 4: 96–101
- Caversaccio M, Romualdez J, Vaecgker R M, et al. Valuable use of computer-aided surgery in congenital bony aural atresia. J Laryngol Otol 2003; 117: 241–8
- Kemppainen H O, Puhakka H J, Laippala P J, et al. Epidemiology and aetiology of middle ear cholesteatoma. Acta Otolaryngol (Stockh) 1999; 119: 568–72
- Cohen D, Tamir D. The pathogenesis of middle ear pathologies in Jerusalem school children. Am J Otol 1989; 10: 456–9
- Metson R B, Cosenza J M, Cunningham M J, Randolph G W. Physician experience with an optical image guidance system for sinus surgery. Laryngoscope 2000; 110: 972–6
- Schlaier J, Warnat J, Brawanski A. Registration accuracy and practicability of laser-directed surface matching. Comput Aided Surg 2002; 7: 284–90
- Fried M P, Kleefield J, Gopal H, et al. Image-guided endoscopic surgery: results of accuracy and performance in a multicenter clinical study using an electromagnetic tracking system. Laryngoscope 1997; 107: 594–601
