Abstract
Pericardial puncture is the percutaneous insertion of a needle into the pericardial space to drain a pathological pericardial effusion. The challenge for the operating surgeon is to reach percutaneously a target zone in the vicinity of the mobile heart, in a soft-tissue environment. The surgeon's ability to accomplish this depends on his own mental picture of the effusion. CASPER is a navigation software using an optical localizer which assists the surgeon by enhancing the representation of the effusion and guiding the needle's progress. Using a localized and calibrated echographic probe, the surgeon acquires a set of images in the region of interest. This zone is then manually segmented on each image, a common zone is computed, and the surgeon defines a trajectory for the needle. During the puncture procedure, the surgeon follows the position of the localized needle on a computer monitor. After initial validation on an experimental phantom, a feasibility study was performed using canine and porcine models. The optical localization device was changed from an Optotrak™ to a Polaris™ device for easier use in the clinical setting. Prior to clinical application, various tests were performed concerning the mobility of the thoracic cage, the reproducibility of the thoracic position over several apneas, and the stability of anatomic structures relative to the thoracic cage. Finally, a first clinical application was successfully performed using this system. The present paper reports on these last two stages.
Introduction
The heart is contained by the pericardium, a fibro-serous membrane in two layers: the visceral and parietal pericardia. The pericardial cavity is a virtual cavity between these two layers that contains a small quantity of serous fluid. This fluid facilitates the movements of the heart. In pathological situations, effusions of liquid may occur, either spontaneously or following a cardiac operation. Because the pericardium is rigid, these effusions induce an increase in the intra-pericardial pressure on the cardiac cavities, leading to a tamponade, a compression of the heart that is potentially lethal. The conventional pericardial puncture Citation[1] performed under echographic control is relatively safe and effective for cases involving large pericardial effusions. However, this technique remains difficult and must often be performed blind, thereby risking failure or accidental puncture of organs (heart, liver, or lung) at a rate dependent on the operating surgeon's experience Citation[2]. The surgeon's ability to accomplish the puncture successfully depends on his own mental picture of the effusion. As in other computer-assisted medical interventions, a computer-assisted system can enhance that representation. First, the representation of the spatial position of the organ is enhanced by image-based localization systems Citation[3]. The motion of the organ due to respiration can then be tracked Citation[4] or simulated Citation[5], and its deformation behavior modeled using a mechanical model of its structure Citation[6].
Our CASPER (Computer ASsisted PERicardial puncture) system is a B-mode ultrasound image-based navigation system. The set of echographic images of the effusion acquired by the surgeon is manually segmented and computed to define a safe target during cardio-respiratory movement. As the probe is located in space by an optical localizer, the surgeon can select the target and trajectory of the needle and precisely insert the localized needle towards this target. The system itself has been described previously: an initial validation was conducted on an experimental model, a dynamic phantom that mimicked cardiac movement Citation[7]. Prior to clinical application, validation on an animal model was necessary: a feasibility study was performed using a canine model Citation[8], and an accuracy and reliability analysis protocol was applied to a porcine model Citation[9]. The feasibility of the technique was demonstrated, with an accuracy of at least 2.5 mm. The goal of the present paper is to highlight improvements to the system and present the first clinical results.
Materials and methods
Our approach follows the usual three steps encountered in a computer-assisted medical intervention Citation[10] and has been described previously Citation[11]. The methodology can be briefly outlined as follows:
1. Perception: The first goal is to acquire and record a set of echographic data and to localize each pixel within a 3D absolute reference frame. This is done using a B-mode ultrasound probe localized by a 3D localizer. The first localizer used was the Optotrak™ (Northern Digital, Inc., Waterloo, Ontario, Canada). For easier use in a clinical context, a Polaris™ localizer (also from Northern Digital, Inc.) was substituted. Polaris™ emits waves in sequence from infrared diodes, which are reflected by reflective dots fixed on the rigid bodies and attached to the ultrasound probe. Polaris™ detects the reflected waves with two linear CCD cameras and localizes them in space in real time by triangulation with an accuracy of approximately 0.3 mm within a volume of 1 m3. Using a calibration procedure for each recorded image, the coordinates of each pixel are computed within an absolute 3D reference frame with an accuracy of 1 mm. After an initial qualitative echographic examination, the physician selects a safe puncture site and acquires a set of almost parallel planes in its vicinity, the ultrasound probe being tracked by the Polaris™ during this acquisition. A referential plane is computed as the median of all the planes and images acquired too far from this referential plane are automatically discarded. The target and trajectory are then defined (see Decision section) and executed (see Action section) in the referential plane.
2. Decision: The aim of this second step is to define the optimal strategy, based on a 2D model of the pericardial effusion and taking into account organ mobility. Determination of an average referential plane is automatically performed by computation, as is the removal of planes beyond a given threshold distance from this reference plane. Thereafter, the behavior of the effusion will be modeled in this plane. The problem of heart motion is solved by finding a target that is stable irrespective of the cardiac cycle, the so-called “stable region”. Determination of this region is performed by manual segmentation of the pericardial effusions' outline in selected views at different points in the cardiac cycle. The various outlines are projected on the referential plane and, after computation by intersection, it is possible to define the stable region. At this stage, both the target (typically in the center of the stable region) and the cutaneous entry point can be selected so that the trajectory will avoid anatomical structures (e.g., the ribs or liver).
3. Action: The third step relies on real-time surgical tool guidance in accordance with a predefined trajectory referring strictly to a digital model. This requires strict immobility between the times of acquisition and puncture. Motion or external deformation is detected by an alarm system consisting of a rigid body () firmly secured to the chest near the puncture area, with real-time tracking by the localizer. This alarm is activated if movement occurs with an amplitude greater than a given threshold, relative to a determined reference position. The surgical tool, in this case a needle, is localized by the localizer. The surgeon is assisted by a passive guidance system based on crosses superimposed on the user interface. This user interface has been improved following preclinical studies Citation[9]. An important point is that this procedure is only used to introduce a J-shaped guide-wire Citation[12].
To make the procedure easier, it is performed during two apneas: one for the echographic acquisition and one for the puncture itself. Obviously, the thoracic cage of the patient must remain in the same position between the two apneas, and it is assumed that the position of the posterior pericardium remains stable throughout, at the same stage of the cardiac cycle. To assess the validity of these hypotheses prior to clinical application, various tests were conducted: First, the mobility of the thoracic cage was assessed under different respiratory states (short apnea, long apnea…) using a rigid body fixed on the anterior aspect of the thorax of a patient whose position was recorded during different respiratory states. Second, the reproducibility of the thoracic position over several apneas was assessed using the same protocol, because the rigid body was considered to be rigidly associated with the thoracic cage. Third, the stability of the posterior pericardium between the two apneas was assessed as follows: Two sets of images were acquired with the same incidence on two successive apneas. From both acquisitions, two images of the heart, one in diastole and the other in systole, were selected, and the posterior pericardium was segmented in each. Finally, to test the beginning of the procedure, real data were collected from volunteer patients with an effusion.
For clinical application, the procedure was carried out according to the following protocol: echographic acquisition was performed in the subxiphoid manner, in apnea during an inspiration state, and the puncture was then made in the same way, also in apnea during an inspiration state. When the target was reached, gentle suction was applied to the syringe connected to the needle. This entire protocol was approved in 2002 by the local institutional human research committee at the Michallon Hospital in Grenoble. The echographic device used was a Hewlett Packard 77025A/S1000 connecting a 7.5-MHz probe to a Matrox 6 acquisition card in a Dell 33-MHz computer. Two passive rigid bodies were fixed to the probe and needle, and were localized by the Polaris™ optical localizer.
Results
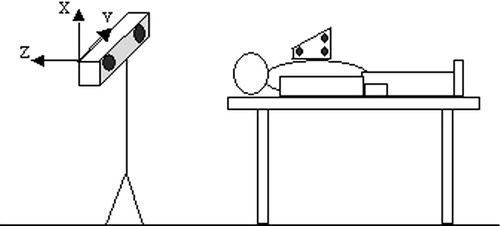
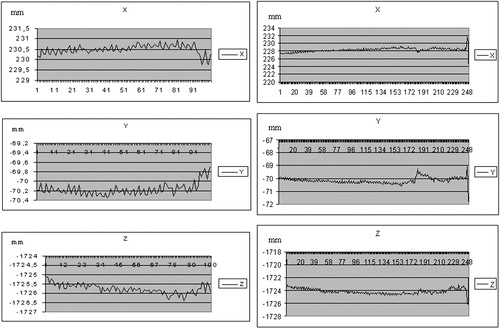
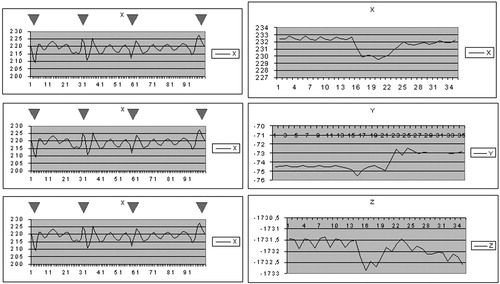
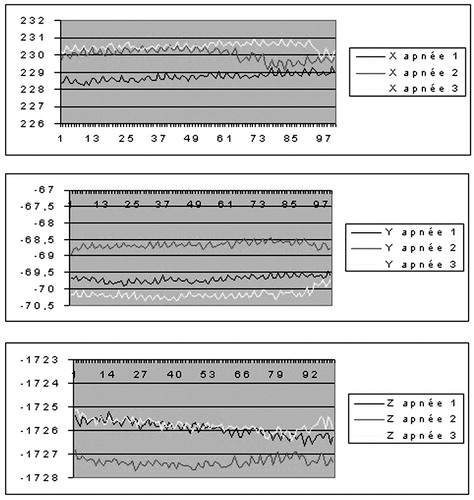
The mobility of the thoracic cage under different respiratory states, assessed on healthy volunteers, showed that the extent of motion along different axes ranged from 2 mm to several cm for normal and deep respiration (). The magnitude was approximately 1 mm for short apnea and 2 mm for long apnea, where the variation was greater at the end of the study, illustrating the fatigue of the subject (). Coughing and movement of the head during normal respiration induced large variations (), resulting in repositioning by several mm.
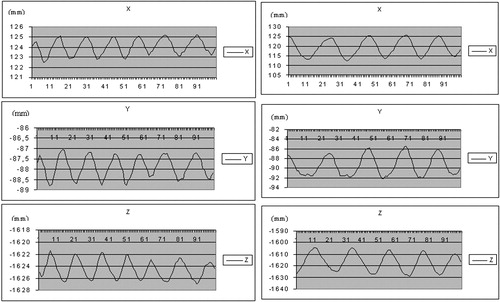
On three successive apneas separated by 5-min intervals, the difference on each axis between the three thoracic cage positions ranged from 0.47 to 1.78 mm ( and ). With regard to the stability of the posterior pericardium between apneas, it was possible to evaluate its relative stability visually ().
Table I. Changes in chest rigid body position between successive apneas with reference to each axis (in mm).
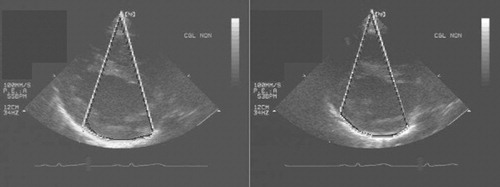
Two patients with an effusion were assessed with the system, demonstrating the possibility of computing a stable zone during apnea as well as during normal respiration ().
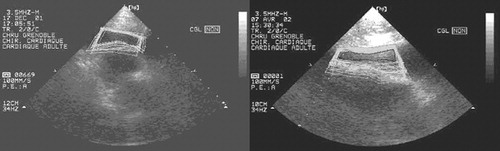
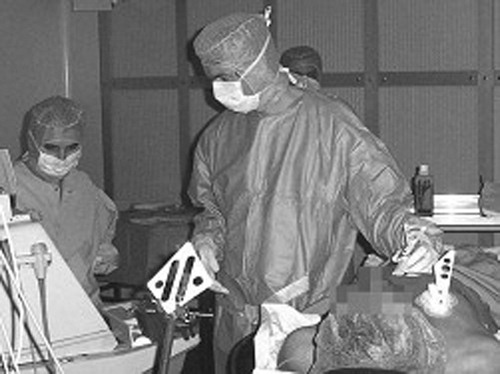
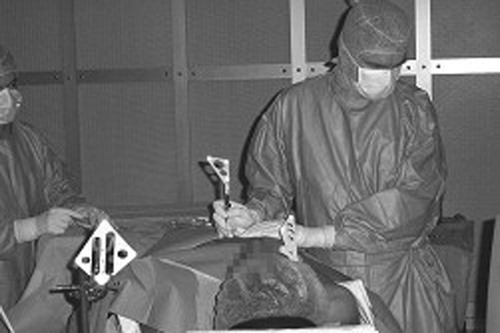
Clinical application of the entire procedure was performed on one patient. A 48-year-old male patient with a pericardic effusion was hospitalized in the cardiology intensive care unit. Echography showed the effusion to be 50 mm wide, and a classic pericardial puncture was undertaken. A volume of 300 ml of liquid was drained with clinical amelioration of the patient, but the effusion did not totally disappear and recurred a few days later. This new effusion was also 50 mm wide. After this relative failure of the classic puncture method, the computer-assisted technique was suggested to the patient, who consented to undergo the procedure once it had been explained to him. As the patient was very cooperative, it was decided to perform the procedure under local anesthesia. Echographic acquisition was performed during a 10-second apnea (). Twenty images were segmented over 3 min, and a stable zone was computed. A target and trajectory were defined by the surgeon, with the distance between the skin and the stable zone being 49 mm. The penetration distance in the stable zone was 6 mm, leaving a security zone of 9 mm. Intra-operative calibration of the needle was easy, with a mean error of 0.15 mm. Guidance () was realized over three successive apneas of 10 s each. The repositioning of the patient's rigid body on the thoracic cage at each apnea was not precise, and the alarm threshold had to be adjusted to 6 mm, an acceptable value in view of the 9-mm security zone. Patient fatigue and the surgeon's difficulty in inserting the needle quickly (during the apnea) increased the difficulty of the guidance step, but the puncture was successful. A volume of 550 ml of liquid was drained, with complete disappearance of the effusion. The duration of the puncture was approximately 2 min. The patient was in the operating room for 1 hr, and the operation lasted 2 hr 30 min for the medical staff.
Discussion
The computer-assisted technique enhanced the surgeon's perception of the effusion and allowed him to perform the puncture procedure more precisely and with greater security. The intrinsic precision of the overall system is limited by the precision of the optical localizer and by the quality of the calibrations of the echographic probe and needle. In contrast, the quality of the modeling of the zone of interest is under the surgeon's control. The image of the zone of interest is bi-dimensional and could appear large in one dimension but small in the perpendicular dimension. The image acquisition is not continuous, and the set of images should sample enough of the cardiac and respiratory cycles so as not to lose information. The cardio-respiratory state should subsequently be reproducible during the guidance phase. The acquisition, modeling and guidance are not independent of each other, and the overall robustness of the system is dependant on the surgeon.
The uncertainty of equivalence between the effusion situations at the times of modeling and guidance could be improved. Ideally, the acquisition should reflect the real situation at the time of guidance and various solutions permitting this could be devised. First, it is now possible to use a flat 3D echographic probe positioned on the thoracic cage of the patient. A volume of interest would be defined by the surgeon and a semi-automatic segmentation algorithm would continuously compute a real-time fix volume. In the center of this interventional probe, a guide-hole would allow the needle to be guided through the volume to the target. Second, for a loculated effusion in which a fix zone does not exist, a realistic mechanical model would be matched to the patient, and monitored inputs of the heartbeat and respiratory flow would activate this model. A robot could be then synchronized to the motion of the effusion. Also, for some very small effusions, the model should simulate deformations arising from the insertion of the needle itself. However, even a standard 3D probe remains an expensive item. In addition, the processing of such a large quantity of image data would have to be made simple enough to be performed in real time and be usable in routine practice. Aspects of the mechanical modeling are also far from resolved. With CASPER, the position of the patient is measured at the moment of acquisition and compared with the position at the moment of guidance. If the difference is greater than an adjustable threshold, an alarm is automatically activated and halts the procedure. This methodology is safe, as we demonstrated in tests conducted before the operation, but it could also prove limiting for a number of indications. The first use of CASPER under clinical conditions (given the inevitable learning curve) has been a success, but further improvements are necessary prior to more widespread use of the system in clinics.
Acknowledgments
The authors would like to thank Mr Eric Boidard for providing the photographs and Mr. Christopher Plaskos for editing the manuscript.
References
- Tsang T, Freeman W, Sinak L, et al. Echocardiographically guided pericardiocentesis: evolution and state-of-the-art technique. Mayo Clin Proc 1998; 73: 647–52
- Pandian N G, Brockway B, Simonetti J, et al. Pericardiocentesis under two-dimensional echocardiographic guidance in loculated pericardial effusion. Ann Thorac Surg 1998; 45: 99–100
- Leroy A, Mozer P, Payan Y, Richard F, Chartier-Kaastler E, Troccaz J. Percutaneous renal puncture: requirements and preliminary results. Surgetica 2002; 303: 309
- Nakamoto M, Sato Y, Miyamoto M, (2002) 3D ultrasound system using a magneto-optic hybrid tracker for augmented reality visualization in laparoscopic liver surgery. Lecture Notes in Computer Science. Proceedings of 5th International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI 2002), TokyoJapan, September, 2002, T Dohi, R Kikinis, et al. Springer, Berlin, 2488: 148–155
- Cleary K, Banovac F, Levy E, Tanaka D. Development of a liver respiratory motion simulator to investigate magnetic tracking for abdominal interventions. Proceedings of SPIE Medical Imaging. 2002
- Leroy A, Payan Y, Voirin D, et al. Finite element model of the liver for computer-assisted hepatic tumor ablation. Proceedings of 5th International Symposium on Computer Methods in Biomechanics and Biomedical Engineering. 2001
- Barbe C, Carrat L, Chavanon O, (1996) Computer assisted pericardic surgery. Computer Assisted Radiology. Proceedings of the International Symposium on Computer and Communication Systems for Image Guided Diagnosis and Therapy (CAR '96), Paris, June, 1996, H U Lemke, M W Vannier, K Inamura, A G Farman, et al. Elsevier, Amsterdam, 781–86
- Chavanon O, Barbe C, Troccaz J, Ribuot C, Blin D. Computer assisted pericardial puncture: work in progress. Comput Aided Surg 1997; 2: 356–64
- Chavanon O, Barbe C, Troccaz J, et al. Accurate guidance for percutaneous access to a specific target in soft tissues: preclinical study of computer-assisted pericardiocentesis. Laparoscop Adv Surg Tech 1999; 9: 259–66
- Cinquin P, Bainville E, Barbe C. Computer assisted medical interventions. Passive and semi-active aids. IEEE Engin Med Biol 1995; 14: 254–63
- Chavanon O, Carrat L, Pasqualini C, et al. Computer-guided pericardiocentesis: experimental results and clinical perspectives. Herz 2000; 25: 761–8
- Stewart J R, Gott V L. The use of a Seldinger wire technique for pericardiocentesis following cardiac surgery. Ann Thorac Surg 1983; 35: 467–8