Abstract
Objective: We present results from the first randomized controlled trial of human vs. telerobotic access to the kidney during percutaneous nephrolithotomy.
Methods: To compare (a) human with robotic percutaneous needle access and (b) local robotic with trans-Atlantic robotic percutaneous needle access, we used a validated kidney model into which a needle was inserted 304 times. Half the insertions were performed by a robotic arm and the other half by urological surgeons. Order was decided randomly except for a sub-group of 30 trans-Atlantic robotic procedures that were controlled by a team at Johns Hopkins, Baltimore, via four ISDN lines.
Results: All attempts were successful within three passes with a median time of 35 s for human attempts compared with a median of 57 s for robotic attempts. The robot was slower than the human to complete insertions (p < 0.001, Mann–Whitney U test), but was more accurate when compared with human operators as it made fewer attempts (88% robotic vs. 79% human first attempt success; p = 0.046, chi-squared test). Times for trans-Atlantic robotic needle insertion (median = 59 s) were comparable to times taken for local robotic needle insertion (median = 56 s) with no difference in accuracy.
Conclusion: Telerobotics is an accurate and feasible tool for future minimally invasive surgery.
Introduction
At a time when technology allows us to transfer information at great speeds across large distances and the public's demand for the best treatment abounds, it is hoped that in the future super-specialized surgeons will operate on their patients, wherever they are in the world. Since the invention of the telephone in 1876, it has been possible to quickly transmit medical information over a significant distance, and more recently video and internet links have enabled surgeons in one hospital to interact directly with patients in another hospital. In its simplest forms, this involves observing a procedure from a distant location (teleproctoring) and then responding to it (teleconsultation) Citation[1]. Taken a step further and using two-way transmission of video and audio signals, the result is distant real-time operative direction and teaching, and this has become known as telementoring or telediagnostics Citation[2], Citation[3]. Since the arrival of the robot and its adaptation to be able to provide surgical assistance from a remote site if needed, the concept of telerobotics or telesurgery has come closer to reality. From this, it has been postulated that robots may be able to perform complex surgical tasks better than their human counterparts, while controlled from another location.
To perform telerobotic surgery, one must have a robot, a means of transmitting the information, a data input centre and a computer at each end that is capable of quickly evaluating the commands given to it and passing them to the operator or robot. The vital ingredient for successful telerobotic surgery lies in the speed of transfer of information from operator to robot and back again Citation[4]. Time delay can significantly affect remote surgical performance; and if the lag time (operator-robot-operator) is >700 ms an operator is unable to learn to compensate. With current high-speed telephone connections, the delay is reduced to only 200–300 ms.
In the last few years, there have been a number of isolated reports of robots performing surgical tasks, some of them operating from different countries Citation[5], Citation[6]. The first telerobotic operation was by an Italian group, headed by Professor Rovetta from Milan, who successfully performed a telerobotic prostate biopsy in 1995 Citation[7]. The dream of having a surgeon in one country performing an operation in another via a computer-assisted link became reality in 2001, when Professor Jacques Marescaux in New York performed a laparoscopic cholecystectomy on a French patient in Strasbourg (The Lindbergh Operation) Citation[8]. Despite these remarkable cases, there has never been a randomized controlled trial comparing the direct performance of man against machine.
We felt that, in order to perform a randomized trial comparing the performance of a robot and a human, percutaneous renal access as the first step in percutaneous nephrolithotomy (PCNL) would be extremely suitable. PCNL is a procedure that involves placing a needle percutaneously into a kidney containing large or multiple calculi. A guidewire is passed and the needle tract is serially dilated to allow passage of a working sheath, through which instruments can be passed. The stone is then fragmented using ultrasonic lithotripsy and the fragments removed. PCNL demands precise needle access to a chosen calyx as its first and most important step. A randomized robotic trial on patients with statistical power to detect modest differences between the two methods of insertion (around 300 procedures) would be unlikely to receive ethical approval in the current climate of increasing litigation. The option of using and sacrificing large numbers of animals (pigs) is also expensive, unattractive, morally difficult to justify, and not legally acceptable in the UK. Our answer was to commission a kidney model adapted from a percutaneous kidney trainer (Limbs and Things, Bristol, UK). Following operator validation of the model, we were able to conduct a randomized controlled trial without harm to patients or animals.
Materials and methods
The model
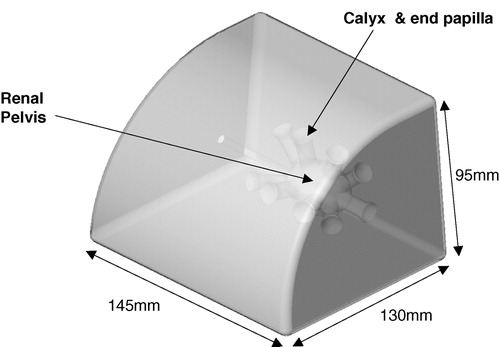
The model for the trial was initially designed as a percutaneous kidney trainer and specially adapted according to our needs. It is constructed as life size (scale 1:1) and, in the training version, the model can be opened posteriorly to introduce artificial calculi. In order to hold contrast for this trial, the models were sealed posteriorly following production. The model is made of a silicone composite and contains a cast of a renal collecting system (40 × 30 × 25 mm3) with 10 calyces (three pairs posteriorly and two pairs anteriorly, diameter 12 mm, length 22 mm) and a ureter. The collecting system contains six calculi that can be positioned individually and the entire renal pelvis (now sealed) can be filled with radio-opaque contrast media. The ureter can be catheterized and contrast medium (Omnipaque/iohexol, Amersham Health, UK) instilled through it into the collecting system. Around the basic slab, a foam-latex ‘skin’ (thickness 1.5 mm) is placed to increase validity (texture and resistance) and prevent direct visualization of the kidney. Removal of the ‘skin’ permitted evaluation of needle position and guidewire insertion. Following each insertion, the local operators assessed successful needle entry by introducing contrast under fluoroscopic control and then a standard guidewire. There were no technical differences between insertions into an empty calyx or one containing a stone. The model, named the ‘Phantom’, looks very similar to a human kidney on fluoroscopy screening. Each model was used for approximately 50 punctures and a total of six identical models were required during the trial ().
The robot
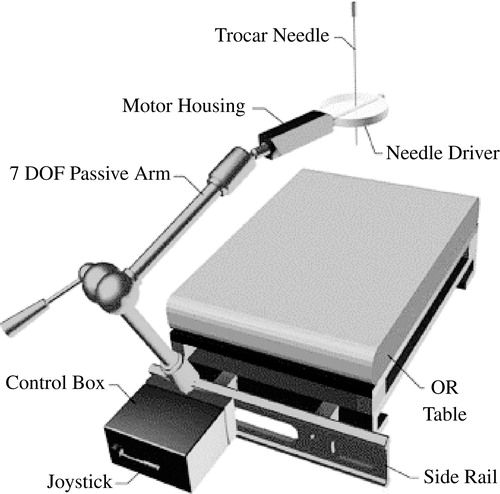
The robot used for this trial was the PAKY-RCM (Percutaneous Access to the Kidney) robot (). This has been developed at the Urobotics Laboratory of the Johns Hopkins University, Baltimore. The device is patented by Johns Hopkins Citation[9], Citation[10] and has been specifically designed and tested in percutaneous surgery Citation[11–13]. The robot has motion that incorporates three degrees of freedom and is mounted directly onto the operating table through a seven-passive-degrees-of-freedom positioning arm Citation[14–16]. The needle driver is radiolucent with only the needle seen on fluoroscopic imaging. This allows the operating surgeon to manipulate the robot into position using a clear X-ray image before driving the needle into the target. The robot has performed two percutaneous needle insertions in Italy while being controlled from Maryland Citation[2].
The trial
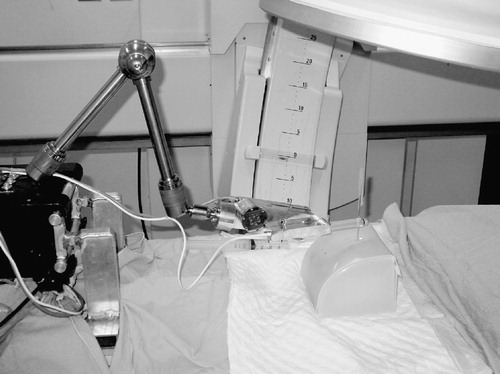
The trial was performed in our endo-urology suite at Guy's Hospital and a high-speed connection to Baltimore was established via four specifically placed ISDN lines with a bandwidth of 512 kb/s. The images were projected in a smooth and continuous manner with a frame speed of 30 frames per second and a delay/lag time of 0.3 s. The total cost for the ISDN line connection over the 5 h of continuous linkup was $5000 ($3000 for the Comstation and $2000 for line rental from British Telecom). Locally, the robot was controlled by five UK-based endourologists. Operator 1 was a trainee, whereas the other four operators were experienced endourologists with significant experience of PCNL. The robot was manipulated locally from a computer station ∼5 m away from the model, with the operator viewing the model, the robot and fluoroscopy on real-time computer screens. Each operator had never previously used the model or controlled the robot in trial conditions, but the Baltimore team had been sent a model prior to the trial to enable model validation. The trans-Atlantic insertions were performed by three endourologists from Baltimore of similar expertise, who had inspected the model and used the robotic arm but had never previously used either in experimental conditions. The human insertions were performed by the UK urologists alternating human with robotic insertions, with the order and target calyx being decided randomly by the toss of a coin. The control group for the trans-Atlantic robotic insertions was performed by one of the UK urologists after the trans-Atlantic phase ().
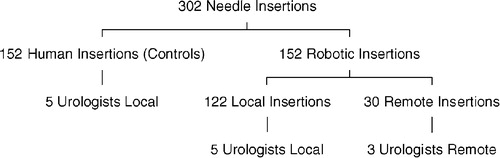
To have 90% statistical power to detect a difference in first-time success rates of 15% (75 vs. 90%) between the two groups using a significance level of 0.05, there should be at least 146 procedures in each arm. In the study, 152 robotic needle insertions and 152 human needle insertions were performed ( and ). A sub-group of 30 needle insertions were controlled entirely by the Johns Hopkins team.
Successful insertion was defined by the Kellet™ needle piercing a papilla (end cup) of a pre-determined calyx to such an extent that a standard guidewire could be easily inserted or contrast instilled into the renal pelvis by the local operators. Glancing needle insertions or insertions up against the far calyceal wall were rejected.
Statistical analysis
Model validation
To test the suitability of the model to accurately represent a human kidney, three US and three UK operators scored it according to a number of variables including authenticity, feel and degree of difficulty. Each variable was scored from 1 to 10 (least to most valid). None of the clinicians involved has any financial interests in the model or the company that produces it.
The median score for all variables was close to 8, indicating a reasonable level of consistency between the model and a human kidney (). When analysed using Kendall's coefficient of concordance, a value of 0.239 (p = 0.013) was obtained. This is the only fully validated percutaneous model that provides the same degree of difficulty for both human and robotic procedures. It was validated using the following criteria:
Authenticity: does it look like a kidney? (general/overall, macroscopic view, radiographic view, computerized view)
Does it feel like a kidney? (generally, texture, resistance, entry into calyx)
Does it offer the same degree of difficulty to human and robot?
Would you use it as a training tool?
Would you recommend it to your residents?
Table I. Phantom validation: scored 1–10.
Analysis of the trial
The data were analyzed by a statistician who was not present for the experiments and was blinded to the groupings in the data (human/robot) so as to eliminate bias. The endourologists, who collected the data, were not involved in the data analysis.
Median insertion times were calculated for operator and method of insertion along with 95% confidence intervals. First attempt success percentages were similarly compared, with exact 95% confidence intervals being calculated.
A Mann–Whitney U test was used to test the significance of differences between times for robotic needle insertion and human needle insertion. The chi-squared test was used to test the accuracy data (one vs. two or more passes). Multivariable linear regression analysis was performed on the time data and multiple logistic regression (one vs. two or more passes) on the accuracy data. For both regression analyses, explanatory variables were selected in a forward stepwise manner. Analyses were carried out using Stata, Release 7.0 (Statacorp, 2001).
Results
All needle insertions (robot = 152 and human = 152) were completed successfully with a maximum of three needle passes (redundant). The interquartile range for the time taken in human attempts was 25–52 s (median 35 s), compared with an interquartile range of 41–80 s (median 57 s) for the robotic attempts (p < 0.001). The robot inserted the needle successfully on the first attempt 88% of times, compared with the human operator who succeeded first time in only 79% of attempts (p = 0.046, chi-squared test).
All operators had a greater or equal first attempt rate for the robotic insertion as compared with the manual insertion, although apart from the most junior operator (number 1 in ), human manual insertion was quicker. When looking at the data, it is seen that operator 5 performed only two trials in each arm of the study. This was due to unforeseen circumstances that prevented him from continuiung the experiment. We have included this data for completeness, but removing it does not alter any of the statistically significant results. The breakdown for median time per procedure and for percentage first attempt success is shown in .
Table II. Results for human against robot.
The robot was noticeably quicker when accessing the lower calyx (median time 49 [lower] vs. 59 [middle] vs. 60.5 [upper] seconds: p = 0.0958, Kruskal–Wallis one-way analysis of variance). There was no difference in accuracy between human and robot for the upper calyx (79% human vs. 78% robot first attempt success: p = 0.878), with the greatest difference being for the lower calyx (80% human vs. 95% robot first attempt success).
In the multivariable linear regression analysis of insertion time, the main explanatory variable was method of insertion, with times for robotic insertion being significantly longer (p < 0.001). This time difference between the two methods was much smaller for the lower calyx compared with the other two calyces (p = 0.003). One of the operators was found to be significantly slower after adjusting for these factors (p = 0.001).
The logistic regression analysis of accuracy data showed that robotic insertion was more accurate (p = 0.006), although this difference was absent where the upper calyx was concerned, confirming the univariate finding mentioned earlier.
Trans-Atlantic analysis
Of the 30 robotic procedures conducted from Baltimore, all were successful within two passes and the time for proven access was 10–182 s (median = 58.5 s). When compared with the 122 local robotic procedures with a time range of 8–220 s (median 56 s), there is no statistical difference between the groups (p = 0.602). With regard to accuracy, the trans-Atlantic group took 1.17 (83% first pass success) passes per successful puncture compared with 1.15 (91% first pass success) for the local robotic group, with no significant difference between them (p = 0.441).
If the trans-Atlantic robotic insertions are compared with the local human insertions (30 controls as in ), the local human trials have a time for access of 15–80 s (median 28 s) and an accuracy of 1.3 (77% first pass accuracy). The robot is slower (p < 0.001 Mann–Whitney U test), but again there is no difference in accuracy (p = 0.519) between the local and the trans-Atlantic group.
Summary of statistical analysis
The significant findings from this trial are
Robotic insertion is slower than human insertion. Median 35 vs. 56.5 s, p < 0.0001;
Robotic insertion is more accurate than human insertion. 79% (first attempt success) vs. 88% (first attempt success), p = 0.046;
The robot performs equally locally and trans-Atlantically. Time: 56 s (locally) vs. 58.5 s (trans-Atlantic), p = 0.602. Accuracy: 89% (locally) vs. 83% (trans-Atlantic), p = 0.441.
Discussion
PCNL was first described by Fernstrom and Johanson in 1976 Citation[17], and the technique rapidly found a place in the management of large renal stones in major stone units worldwide. This trial supports the use of robotic techniques both locally and remotely for percutaneous access as the first step in PCNL. The placement of the Kellet needle (Boston Scientific Ltd., UK) in PCNL Citation[18] is the most technically difficult and important step in gaining access to the desired calyx before subsequent tract dilatation and stone extraction. Several unsuccessful attempts at needle insertion would increase the chance of patient morbidity in the form of haemorrhage, post-operative pain and damage to adjacent structures. Hence, it is an ideal procedure to be performed robotically in an attempt to improve accuracy and decrease the risk of morbidity to the patient. In addition to these benefits to the patient, telerobotic surgery, whether local or trans-Atlantic, can minimize or eliminate radiation exposure for the operating surgeon.
There have been no randomized controlled trials in medical robotics to date, with only sporadic cases or trials of limited size being reported, which is almost certainly due to the difficulty in recruiting patients Citation[19]. Experimentation performed on pig models has found that, although potentially feasible, the low position of the animal's ribcage often interferes with percutaneous robotic access Citation[1].
The robotic arm used in this trial has already been tested on patients in Baltimore with successful results that have led to the conclusion that it is a safe and reliable method for obtaining percutaneous renal access Citation[2]. However, the numbers are small and the data non-randomized.
Our data indicate that a robot can perform a precise task in a comparable way to a human operator. The data also support the view that remote surgery is not just a futuristic ideal, but also a genuine and feasible future development. The data show that although the robotic-assisted access is slower than that of the surgeon's hand, it is more accurate as it makes fewer passes to hit the target. This is because, unlike the human hand, the robotic arm has no tremor and can accurately perform the given task once its trajectory has been set.
The figures for the remote trans-Atlantic access show no difference in the robotic performance over a larger distance either in terms of time or accuracy when compared with both the local robotic and the local human insertions. These results are equivalent whether the robotic arm is controlled from a distance of 5 m or 5000 miles. One should, of course, bear in mind the other issues surrounding remote surgery such as the reliability of the ISDN lines and the subjects of consent and responsibility for the patient. Although we did not have any communication problems with the remote access, a local team of surgeons with sufficient experience to carry out each procedure independently should always be available. Using this evidence, strongly supporting remote surgery, it may be possible to perform large numbers of procedures on consenting patients and potential exists for remote surgery on the battlefield or in space Citation[20].
The difference in accuracy between individual calyces may have been due to the slightly differing angles of the lower calyx compared with others. The lower calyx is more directly in line with the angle of needle approach compared with the upper calyx, which is more perpendicular. The same is true in clinical practice, where lower pole access is generally easier than upper pole, supracostal access during PCNL. Operator 1, the only trainee, was the only operator to have quicker times and better accuracy with the robot when compared with the human insertions. This suggests that the PAKY robot may help to overcome the learning curve for percutaneous access.
It would appear that the main disadvantage of the model, when compared with a human subject, is the lack of simulation of renal movement seen during normal respiration. This factor is nullified in the trial, as it remains constant during both human and robotic accesses. It should be noted that respiration is often slowed or stopped momentarily at the moment of initial needle insertion in several centres. However, a virtual reality model to simulate respiration is being planned. The validators of the model felt that the resistance of the synthetic skin was less than that of a patient and that, by its nature, there was a uniform depth to the kidney for each procedure, which varies widely in the clinical setting.
The future
Robotic surgery is set to become the next major revolution in modern surgery, with remote operative control becoming an increasingly significant part of this development. This study has confirmed that robotic access is a feasible, reproducible, and technically achievable goal, especially when performing procedures that demand precision. The robotic needle insertion has been seen to be slightly slower than manual insertion, but outperforms the human operator in terms of increased accuracy. Trans-Atlantic telerobotic access is as accurate as local telerobotic access.
The ultimate aim is to use this data to gain permission to perform a randomized control trial on patients with sufficient power to allow meaningful interpretation of the results. In the short term, it is hoped that a virtual reality simulator can be developed, which will be able to move in a fashion analogous to human respiration, thus increasing model validation. It is also anticipated that a similar trial involving the same model and robot can be performed from a different country, reproducing our results.
Acknowledgements
The sources of funding for this study were Guy's Hospital and Johns Hopkins Research Funds. Model manufacturer and fluoroscopy equipment were provided by Limbs and Things, Bristol, UK, and Richard Harris, Philips, respectively. We thank British Telecom for the ISDN line connection and Mr Fred Compton (statistical assistance) and Miss Marie Gwyn-Jones (radiographer) for their assistance.
Disclosure
Under a licensing agreement between Image Guide and the Johns Hopkins University, Dr Kavoussi and Dr Stoianovici are entitled to a share of any royalties received by the University on sales of products described in this article. Dr Kavoussi, Dr Stoianovici and the University own Image Guide stock, which is subject to certain restrictions under University policy. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
References
- Cadeddu J. A., Stoianovici D., Kavoussi L. R. Robotic surgery in urology. Urol Clin North Am 1998; 25: 75–85
- Link R. E., Schulam P. G., Kavoussi L. R. Remote monitoring and assistance during laparoscopy. Urol Clin N Am 2001; 28: 177–188
- Micali S., Virgili G., Vannozzi E., Grassi N., Jarrett T. W., Bauer J. J., Vespasiani G., Kavoussi L. R. Feasibility of telementoring between Baltimore (USA) and Rome (Italy): the first five cases. J Endourol 2000; 14: 493–496
- Fabrizio M. D., Lee B. R., Chan D. Y., Stoianovici D., Jarrett T. W., Yang C., Kavoussi L. R. Effect of time delay on surgical performance during telesurgical manipulation. J Endourol 2000; 14: 133–138
- Janetschek G., Bartsch G., Kavoussi L. R. Transcontinental interactive laparoscopic telesurgery between United States and Europe. J Urol 1998; 160: 1413
- Dasgupta P. Robotics in urology. BJU Int 2001; 88: 300
- Rovetta A., Sala R. Execution of robot-assisted biopsies within the clinical context. J Image Guided Surgery 1995; 1(5)280–287
- Marescaux J., Leroy J., Gagner M., Rubino F., Mutter D., Vix M., Butner S. E., Smith M. K. Transatlantic robot-assisted telesurgery. Nature 2001; 414: 710
- Stoianovici D., Whitcomb L. L., Mazilu D., Taylor R. H., Kavoussi L. R.. Adjustable remote center of motion robotic module. United States Provisional Patent 60/354,656, 2001
- Stoianovici D., Kavoussi L. R., Whitcomb L. L., Taylor R. H., Cadeddu J. A., Demaree R. D., Basile S. A.. Friction transmission with axial loading and a radiolucent surgical needle driver. United States Patent 6,400,979, June 4, 2002
- Cadeddu J. A., Bzostek A., Schreiner S., Barnes A. C., Roberts W. W., Anderson J. H., Taylor R. H., Kavoussi L. R. A robotic system for percutaneous renal access. J Urol 1997; 158: 1589–1593
- Stoianovici D., Cadeddu J. A., Demaree R. D., Basile H. A., Taylor R. H., Whitcomb L. L., Sharpe WN Jr, Kavoussi LR. (1997) An efficient needle injection technique and radiological guidance method for percutaneous procedures. Lecture Notes in Computer Scienc. Proceedings of First Joint Conference on Computer Vision, Virtual Reality and Robotics in Medicine and Medical Robotics and Computer-Assisted Surgery (CVRMed–MRCAS'97, GrenobleFrance, March, 1997. Springer, Berlin, 1205: 295–298
- Stoianovici D., Cadeddu J. A., Demaree R. D., Basile H. A., Taylor R. H., Whitcomb L. L., Kavoussi L. R. A novel mechanical transmission applied to percutaneous renal access. Proceedings of the ASME Dynamic Systems and Control Division, American Society of Mechanical Engineers—Winter Annual Meeting, (DSC Vol. 61. November 17–18. DallasTX, USA 1997
- Stoionovici D., Whitcomb L. L., Anderson J. H., Taylor R. H., Kavoussi L. R. (1998) A modular surgical robotic system for image guided percutaneous procedures. Lecture Notes in Computer Scienc. Proceedings of First International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI'98), Cambridge, MA, October, 1998. Springer, Berlin, 1496: 404–410
- Stoianovici D. URobotics—urology robotics at Johns Hopkins. Comput Aided Surg 2001; 6: 360–369
- Solomon S. B., Patriciu A., Bohlman M. E., Kavoussi L. R., Stoianovici D. Robotically driven interventions: a method of using CT fluoroscopy without radiation exposure to the physician. Radiology 2002; 225: 277–282
- Fernstrom I., Johanson B. Percutaneous pyelolithotomy: a new extraction technique. Scand J Urol Nephrol, 1976; 10: 257–259
- Wickham J. E.A., Kellett M. J. Percutaneous nephrolithotomy. Br Med J 1981; 283: 1571–1572
- Su L-M., Stoianovici D., Jarrett T. W., Patriciu A., Roberts W. W., Cadeddu J. A., Ramakumar S., Solomon S. B., Kavoussi L. R. Robotic percutaneous access to the kidney: comparison with standard manual access. J Endourol 2002; 16: 471–475
- Cubano M., Poulose B. K., Talamini M. A., Stewart R., Antosek L. E., Lentz R., Nibe R., Kutka M. F., Mendoza-Sagaon M. Long distance telementoring: a novel tool for laparoscopy aboard the USS Abraham Lincoln. Surg Endosc 1999; 13: 673–678