Abstract
Among the various EU research projects concerning the medical application of virtual reality, the project Ist-1999-12175, called IERAPSI (Integrated Environment for the Rehearsal and Planning of Surgical Interventions), specifically addressed the creation of a virtual and interactive surgical field for the temporal bone using three-dimensional images derived from CT data. We report on the experience obtained in the IERAPSI project in simulating a canal wall-up mastoidectomy. A surgeon with extensive experience in surgery of the petrous bone performed the mastoidectomy. The operative field included the mastoid, with its substantial differences in density between the cortex and the pneumatized bone, together with soft tissue structures, both on the border and inside the bone. The simulation is better in the first part of the operation than in the second part, suffering from a lack of haptic feedback from soft tissue and the surgical tool in deeper contexts, and under-representation of the variability inherent in pneumatized bone. This said, the excellent representation of dust production and removal, 3D simulation through color, and very good visual and haptic feedback in the early stage of the procedure are impressive. IERAPSI represents a potential surgical planning theater for the training of students and young surgeons, but is also expected to aid expert surgeons in the preoperative planning of difficult cases.
Introduction
The temporal bone, constituting the wider bony portion of the cranial base, contracts relationships with the internal carotid artery, the jugular vein, the cerebral cortex, and some cranial nerves, of which the facial nerve lies in particularly close proximity. The bone also contains tiny structures that are amongst the most sophisticated and delicate in the human body: the cochlear and vestibular apparatuses and the ossicular chain Citation[1]. Due to these anatomical features, ear surgery introduces numerous risks of damage.
A very common surgical procedure in the temporal bone is mastoidectomy. This consists of the removal of the aerial cavities in the mastoid process and is performed to create an opportune surgical access to the middle or inner ear. There are two types of mastoidectomy: in the canal wall-up type, the posterior wall of the external auditory canal is left intact, while the canal wall-down mastoidectomy involves its complete removal. During the procedure, the surgeon must be able to distinguish readily the several structures that are progressively met during drilling Citation[2–5].
High-resolution computed tomography (CT) is established as the ideal method for appraisal of the middle ear and the bony components of the inner ear Citation[6], while magnetic resonance imaging (MRI) is used in the study of the membranous labyrinth, the internal auditory canal and the cerebello-pontine angle Citation[7]. However, the two-dimensional images obtained with these methods do not always supply the information necessary for planning the intervention; a more complete preoperative appraisal is obtained with three-dimensional (3D) views. In particular, the technology of virtual reality permits a more realistic visualization of the 3D structures Citation[8–10]. Among the different EU research projects concerning the medical application of virtual reality, the project Ist-1999-12175, called IERAPSI (Integrated Environment for the Rehearsal and Planning of Surgical Interventions), specifically addressed the creation of a virtual and interactive surgical field for the temporal bone using 3D images derived from CT data Citation[11]. The main goal of the project was to provide an interactive computerized environment for the planning and rehearsal of surgical procedures on the petrous bone in individual patients. The institutions participating in the project were the University of Pisa, Italy; CRS4 – Center for Advanced Studies, Research and Development in Sardinia, Italy; Dresden University of Technology, Germany; University College London, UK; Victoria University of Manchester, UK; Virtual Presence, Ltd., UK; Genias Benelux BV, The Netherlands; and CS Systemes d'Information, Toulouse, France. The project had a three-year duration and focused specifically on producing the following deliverables:
An interactive 3D visualization to allow production of surgical planning datasets.
Segmentation techniques to extract features from datasets based on their imaging characteristics from multiple co-registered sources.
A pre-operative planning visualization tool to view the segmented data.
A physics-based surgical simulation system using haptics and visual feedback for training and surgical planning using individual patient data.
Materials and methods
Three-dimensional virtual surgical field
The 3D virtual surgical field was generated by collecting multirow CT datasets (LightSpeed Plus scanner, GE Medical Systems) in a patient who was a candidate for mastoidectomy. The CT datasets were obtained in the axial plane with collimation of 1.25 mm and row thickness of 0.63 (reconstruction spacing of 0.63 mm), pitch 3, and the algorithm of reconstruction for bone.
The 3D data preparation was performed with volume-rendering software developed at CRS4. Through manual and automatic segmentation techniques, the bone, the meninx, the sigmoid sinus, the jugular bulb, the internal carotid artery, and the air cells of the tympanic cavity were identified and introduced as distinct objects in the virtual operating field.
The software generating the operating field is run on a dual-processor computer (PIII/800 MHz, with 512 MB RAM and an NVIDIA GeForce 4 Ti 4600 graphic card Citation[12]). To increase the 3D conspicuity of the surgical environment, a binocular device (n-Vision VB30) that allowed a stereoscopic view was connected to the PC.
Surgical simulation
Virtual surgical instruments were introduced into the surgical field by adding a second, single-processor computer (P4/1500 MHz with 256 MB RAM). The surgical instruments were designed and introduced into the virtual field in accordance with the real surgical environment. Those manipulated with the right hand comprised a drill burr (4 mm in diameter) that reduced the bone to dust and an irrigator that introduced water into the operating field to clean the tip of the cutter, in the process forming a bone-dust paste by mixing water and bone debris. A sucker for removing the bony paste and blood was controlled by the left hand ().
Figure 1. Virtual operating field. The surgical instruments represented are the sucker (black arrow), the drill burr (white arrow) and the irrigator (black arrowhead).
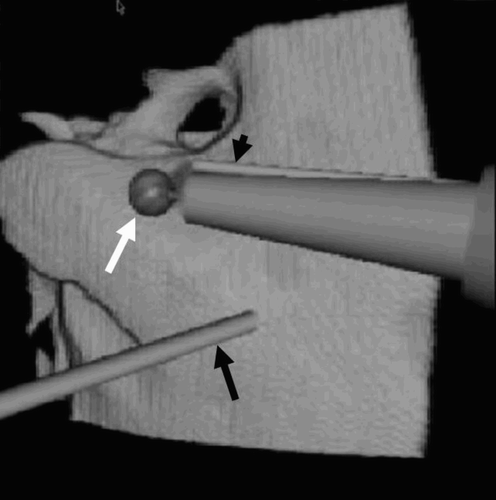
The virtual instruments were controlled by the surgeon's right hand via a Phantom 1.0 haptic device, which allowed movement of the drill burr with six degrees of freedom (DOF), and generated force feedback with three DOF. The surgeon could control the sucker with his left hand by means of a Phantom Desktop haptic device with six DOF, and received force feedback with three DOF Citation[13] ().
Simulation of the mastoidectomy
To test the IERAPSI system, a surgeon (SSF) with extensive experience of interventions on the temporal bone (over 1000 procedures) performed the simulation. He was asked to reproduce the initial phase of a canal wall-up mastoidectomy and evaluate the realism of the system.
Results
The surgeon performed the virtual mastoidectomy using the following external anatomical points of reference: the lateral surface of the mastoid process of the temporal bone; the meatus of the external auditory canal; and the spine of Henle located behind the meatus.
Figure 3. The first action in the mastoidectomy is drilling two lines: one vertical and posterior to the facial nerve canal (black arrow); the other horizontal, along the sigmoid sinus (arrowhead). eac = external auditory canal; t = tip of mastoid; s = spine of Henle.
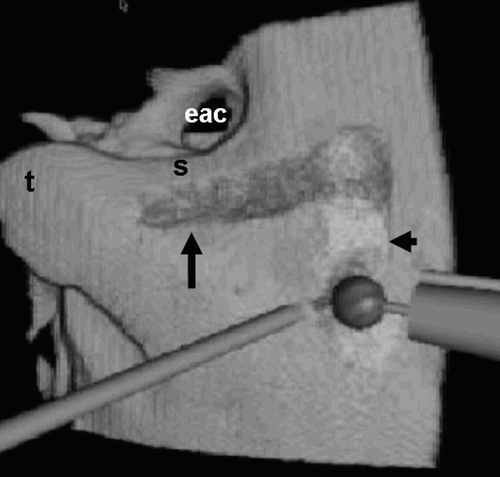
Figure 4. Triangular surgical cavitation of the mastoid (arrows) is used to remove the mastoid air cells and reach the mastoid antrum. The drill burr (arrowhead) is creating access to the tympanic cavity.
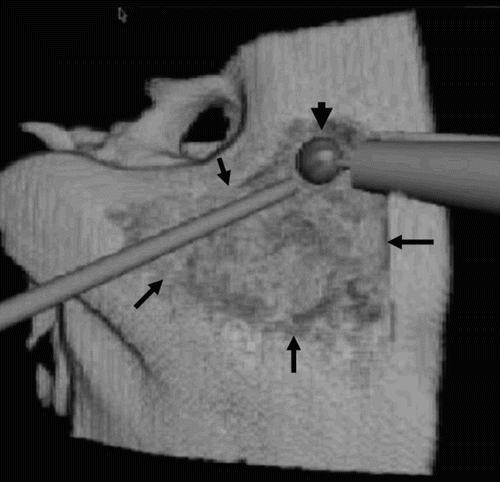
To begin, the mastoid cortex was decorticated by drilling two lines along the vertical segment of the facial nerve and along the plane of the sigmoid sinus. Following this, a straight cut was made along the temporal line, posteriorly, into the sino-dural angle, and a second cut was made perpendicular to the former line immediately posterior to the external canal wall. During the drilling, bleeding was simulated in some instances, and eliminated with the virtual sucker. The deeper excavation in the mastoid process was intended to remove the bone over the sino-dural angle and unroof the sigmoid sinus. The aditus ad antrum tympanicum was then opened to allow access to the tympanic cavity.
The duration of the virtual mastoidectomy was shorter than that of the normal operation, as certain elements (changing the bar, possible complications, etc.) have not yet been included in the simulation.
Figure 5. During the drilling, some bleeding is simulated (arrows). A deeper excavation allows unroofing of the sigmoid sinus (arrowheads). The drill burr is opening the mastoid antrum (white arrow).
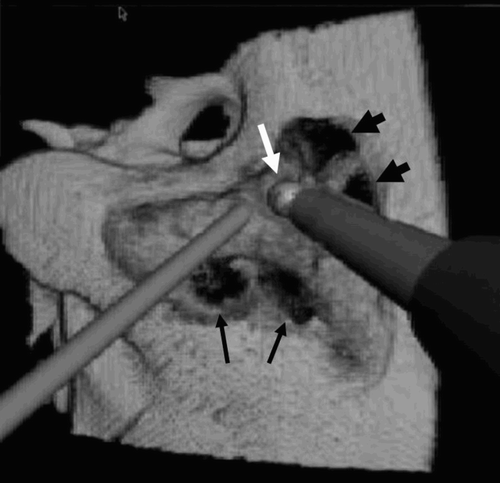
The surgeon's overall impression was very positive: he indicated satisfaction with the reproduction of the bone-cutting procedure and the response of the virtual instruments. In particular, the haptic feedback was judged to be sufficiently realistic. The only minor drawback was the lack of tangential feedback during the drilling procedure. Bleeding and suction were convincingly reproduced.
Discussion
A virtual surgical simulator for temporal bone providing aural, visual and force (haptic) feedback has been reported by Wiet et al. Citation[14]. The 3D environment was generated from imaging data (MR and CT) acquired from fresh (non-perfused) cadavers. Similarly, Bernardo et al. reported the development of a surgical simulator in which spatial anatomical data were recorded from sequentially deeper cadaveric head dissections Citation[15]. All these systems were based on datasets obtained from cadavers, and the simulations were therefore not patient-specific.
The most innovative aspect of IERAPSI was that CT data could be obtained directly from a patient who was a candidate for surgery of the temporal bone. This allowed surgical planning or training to be performed on a patient-specific 3D model, reproducing the real surgical environment as realistically as possible.
A further advantage of the IERAPSI system is the user interface, which comprises aural, visual and tactile feedback. Wiet et al. had previously reported a similar capability, but IERAPSI featured, in addition, simulation of bleeding, bone dust and paste formation during drilling. Such elements have a great impact on surgical performance, as they obscure the surgeon's view and must be constantly removed.
The IERAPSI system served to augment the reality of the surgical simulation, but had some limitations. The original data were obtained from non-enhanced multi-row CT, so there was no representation of the soft tissues of the mastoid region, vessels, and static fluids within the cochlea. Another limitation concerned the facial nerve, as no specific tasks were dedicated to isolate its bony canal and represent it as a distinct object in the 3D volumetric dataset.
Conclusion
Overall analysis of the surgeon's comments suggests that IERAPSI represents a surgical planning tool that can enhance the hands-on training of students and young surgeons. However, IERAPSI may be also expected to effectively aid expert surgeons in the preoperative planning of difficult cases, for which the simulation will be further improved to make it more realistic. The first step in that direction will be the future upgrade of IERAPSI in the simulation of other surgical interventions, such as the positioning of cochlear implants or the removal of an acoustic neuroma.
References
- Gray H. Anatomy of the Human Body. Lea & Febiger;, Philadelphia 1918
- Kanno T, Kiya N, Varghese Sunil M. Microsurgical anatomy of the retroauricular, transcervico mastoid infralabyrinthine approach to jugular foramen. Neuroanatomy 2003; 2: 28–34
- Cass S P. Mastoid surgery. Operative Otolaryngology Head and Neck Surgery, E N Myers, R L Carran, S P Cass, et al. WB Saunders Company, Philadelphia 1997; II: 1280–1298
- Stjernholm C. Aspects of temporal bone anatomy and pathology in conjunction with cochlear-implant surgery. Acta Radiol Suppl 2003; 430: 2–15
- Linstrom C J, Meiteles L Z. Facial nerve monitoring in surgery for congenital auricular atresia. Laryngoscope 1993; 103(4 Pt 1)406–415
- Greess H, Baum U, Romer W, Tomandl B, Bautz W. CT and MRI of the petrous bone. HNO 2002; 50(10)906–919
- Ali Q M, Ulrich C, Becker H. Three-dimensional CT of the middle ear and adjacent structures. Neuroradiology 1993; 35(3)238–241
- Ito T, Naganawa S, Fukatsu H, Ishigushi T, Ishigaki T, Kobayashi M, Kobayashi K, Ichinose N, Miyazaki M, Kassai Yukimori. High-resolution MR images of inner ear internal anatomy using a local gradient coil at 1.5 Tesla: Correlation with histological specimen. Radiation Medicine 1999; 17(5)343–347
- Lopponen H, Holma T, Sorri M, Jyrkinen L, Karhula V, Koivula A, Ilkko E, Laitinen J, Koivukangas J, Oikarinen J, Alamaki O. Computed tomography data based rapid prototyping model of the temporal bone before cochlear implant surgery. Acta Otolaryngol Suppl 1997; 529: 47–49
- Gandhe A J, Hill D L, Studholme C, Hawkes D J, Ruff C F, Cox T C, Gleeson M J, Strong A J. Combined and three-dimensional rendered multimodal data for planning cranial base surgery: a prospective evaluation. Neurosurgery 1994; 35(3)463–470, discussion 471
- Jackson A, John N W, Thacker N A, Ramsden R T, Gillespie J E, Gobbetti E, Zanetti G, Stone R, Linney A D, Alushi G H, Franceschini S S, Schwerdtner A, Emmen A. Developing a virtual reality environment in petrous bone surgery: a state-of-the-art review. Otol Neurotol 2002; 23(2)111–121
- Agus M, Giachetti A, Gobbetti E, Zanetti G, Zorcolo A. A multiprocessor decoupled system for the simulation of temporal bone surgery. Computing and Visualization in Science 2002; 5(1)35–43
- Agus M, Giachetti A, Gobbetti E, Zanetti G, Zorcolo A, Picasso B, Sellari Franceschini S. A haptic model of a bone-cutting burr. Stud Health Technol Inform 2003; 94: 4–10
- Wiet G J, Stredney D, Sessanna D, Bryan J A, Welling D B, Schmalbrock P. Virtual temporal bone dissection: an interactive surgical simulator. Otolaryngol Head Neck Surg 2002; 127: 79–83
- Bernardo A, Preul M C, Zabramski J M, Spetzler R F. A three-dimensional interactive virtual dissection model to simulate transpetrous surgical avenues. Neurosurgery 2003; 52(3)499–505
