Abstract
Objective: A robotic system is presented for flexible needle steering and control in soft tissue. Materials and Methods: Flexible needle insertion into a deformable tissue is modeled as a linear beam supported by virtual springs, where the stiffness coefficients of the springs can vary along the needle. Using this simplified model, the forward and inverse kinematics of the needle are solved analytically, thus enabling both path planning and path correction in real time. Given target and obstacle locations, the computer calculates the needle tip trajectory that will avoid the obstacle and hit the target. Using the inverse kinematics algorithm, the corresponding needle base maneuver needed to follow this trajectory is calculated.
Results: It is demonstrated that the needle tip path is not unique and can be optimized to minimize lateral pressure of the needle body on the tissue. Needle steering, i.e., the needle base movements that steer the needle tip, is not intuitive. Therefore, the needle insertion procedure is best performed by a robot. The model was verified experimentally on muscle and liver tissues by robotically assisted insertion of a flexible spinal needle. During insertion, the position and shape of the needle were recorded by X-ray.
Conclusions: This study demonstrates the ability to curve a flexible needle by its base motion in order to achieve a planned tip trajectory.
Introduction
The trend in contemporary medicine is towards less invasive and more localized therapy. Many routine treatments employed in modern clinical practice involve percutaneous insertion of needles and catheters for biopsy and drug delivery. The aim of a needle insertion procedure is to place the tip of an appropriate needle safely and accurately in the lesion, organ or vessel. Examples of treatments requiring needle insertions include vaccinations, blood/fluid sampling, regional anesthesia, tissue biopsy, catheter insertion, cryogenic ablation, electrolytic ablation, brachytherapy, neurosurgery, deep brain stimulation and various minimally invasive surgeries. In general, complications of percutaneous needle insertion are due to poor technique and needle placement Citation[1]. Physicians and surgeons often rely only on kinesthetic feedback from the tool that they correlate with their own mental 3D perception of anatomic structures. However, this method has significant limitations: as the needle penetrates the tissue, the tissue deforms and thus, even when working with straight rigid needles, the target can be missed Citation[2]. To improve needle placement, rigid needles can be maneuvered under image guidance. Furthermore, Alteroviz et al. Citation[3] have proposed a way to predict rigid needle placement error in prostate brachytherapy procedures and to correct for this error by choosing an alternative insertion point. Despite such advances in rigid needle placement, in some cases the problem persists of rigid needles causing excessive, injurious pressure on tissues.
An alternative approach to ensuring the success of percutaneous procedures is to employ thin and flexible needles. There are numerous advantages to using such needles. According to retrospective studies that examined the relationship between post-biopsy behavior and biopsy needle diameter, less serious complications occur with fine (<1 mm) biopsy needles than with standard coarse needles Citation[4]. Furthermore, thinner needles cause less damage and reduce the chance of Post Dural Puncture Headache (PDPH) appearing after spinal anesthesia; indeed, the relative risk of PDPH decreases with reduction in needle diameter Citation[5]. Moreover, flexible needles facilitate curved trajectories that may be desirable in order to avoid sensitive tissues, such as bone or blood vessels, lying between the feasible entry points and potential targets. However, a major disadvantage to using thin flexible needles is that they are very difficult to control. They have non-minimum phase behavior and do not lend themselves to intuitive (human) control. Solving this problem of control requires development of a computerized robotic system that can plan and perform insertions of thin flexible needles. Creation of such a system represents a challenge for mechanical engineers and roboticists, but is nevertheless an urgent therapeutic goal as it will reduce morbidity and patient suffering following percutaneous procedures.
Devising a method to predict flexible needle motion was first addressed by DiMaio and Salcudean Citation[6]. To solve the inverse kinematics of the needle, the authors used iterative numerical computing of the Jacobian-modeled movement exhibited by a flexible needle. The solution requires nine independent computations for devising the Jacobian elements that account for the two-dimensional (2D) finite element mesh of the tissue and the iterative nonlinear flexion of a beam. A limitation of this work is that, due to the computational complexity, it does not allow for real-time simulation and control of the needle path. An active, flexible steering system using shape memory alloys has been suggested for steering a catheter Citation[7]. However, such a system is not suitable for thin needle navigation because of its size limitations. Several groups have focused on calculating the effects of needle bending. Kataoka et al. investigated needle deflection due to the bevel of the tip during linear needle insertion and expressed the deflection as a function of the driving force Citation[8]. O'Leary et al. demonstrated experimentally that needle bending forces are significantly affected by the presence of a bevel tip Citation[9]. Ebrahimi et al. generated needle bending by incorporating a pre-bent stylus inside a straight canula Citation[10]. Such studies have led to the realization that bevel-tip needles can be steered by taking advantage of needle bending and by exploiting the asymmetric force applied by the needle tip to the tissue. This insight encouraged recent work aimed at steering a highly flexible needle through firm tissue by formulating its motion as a nonholonomic kinematics problem Citation[11–13]. It is notable that the effect of the beveled tip decreases with reduction in needle diameter, as the lateral force is proportional to the area of the tip.
The present investigation describes an algorithm that allows fast path planning and real-time tracking of a flexible needle insertion procedure. The aim of this work is the creation of an image-based system for automatic flexible needle insertion and obstacle avoidance.
Materials and methods
Virtual springs model
In this investigation, modeling the movements of a flexible needle is based on the assumption of quasistatic motion; the needle is in an equilibrium state at each step. It is known that needle deflection due to interactions with biologic soft tissue is nonlinear with strain. However, the work of Simone et al. implies that it is reasonable to assume a linear lateral force response for small displacements Citation[14], Citation[15]. The tissue forces on the needle are modeled as a combination of lateral virtual springs distributed along the needle curve plus friction forces tangent to the needle. Since the tissue elastic modulus changes as a function of strain, we update the coefficients of the virtual springs according to the strain-dependent dynamic elastic modulus and linearize the system at each step. The concept is illustrated in .
Figure 1. Virtual springs model. The interaction of the tissue with the needle is modeled by distributed virtual springs. [Color version available online.]
![Figure 1. Virtual springs model. The interaction of the tissue with the needle is modeled by distributed virtual springs. [Color version available online.]](/cms/asset/b85e3fd9-7bb3-4542-a581-9e7e5e1f87de/icsu_a_189223_f0001_b.jpg)
As the shape of the needle changes, the location and orientation of the virtual springs change accordingly. The linearized system model yields the shape of the needle at each step. There is no physical meaning to the free length of the virtual springs. The only important parameter of a spring is the local stiffness coefficient that expresses the force of the tissue on the needle as a function of local displacement. The stiffness coefficients of the virtual springs are determined experimentally or by using preoperative images assuming empiric stiffness values of tissues and organs Citation[15].
The linearized system solution
Assuming small displacements, the needle is approximated by a linear beam subjected to point forces, as shown in . With appropriate spacing of elements, it can approximate a flexible beam according to the elastic foundation model.
Figure 2. Linear system model. A flexible beam subjected to a number of virtual springs. [Color version available online.]
![Figure 2. Linear system model. A flexible beam subjected to a number of virtual springs. [Color version available online.]](/cms/asset/de14f91d-6a36-455a-b923-0cc4bd89f5ab/icsu_a_189223_f0002_b.jpg)
At each joint, the force applied by a virtual spring is proportional to the displacement of the spring from its initial position:where ki is the virtual spring coefficient, wi the displacement at point i, and w0i the position of freed spring i.
As the forces are a function of the deflection, the needle movement cannot be modeled by treating the beam as one element. Therefore, the beam is split into a number of elements, so that each beam element is subjected to two neighboring forces. Thus, the first element is the part of the needle outside of the tissue, and the rest of the elements are distributed along the inner part according to the level of discretization. Each element behaves as a linear beam subjected to shearing forces at its borders. The displacement of each element is given by a third degree polynomial. We adopt the nodal degrees of freedom from finite elements theory, in which the coordinates are specifically identified with a single nodal point and represent a displacement or rotation, having clear physical interpretation. The displacement y(x) has the form ofwhere N1, N3 are the coordinates and N2, N4 are the slopes at x = 0 and x = l of an element, respectively. ϕi are the shape functions of third degree.
Substituting boundary conditions as displacement and slope at the base and tip of the needle, the result is 4 × n equations: 2 at each side and 4 for each internal node, which yields the global matrix equationwhere K is the matrix of coefficients of Ni,j-elements degrees of freedom, and N is the vector of Ni,j, where i is the element number and j is the degree of freedom of element i.
Forward kinematics
The above solution solves for the translation and rotation of the needle taking into account 2 degrees of freedom (DOF) of the needle base: vertical translation y and slope θ. Applying axial translation, along the x-direction, to the non-compressible needle means that the part of the needle outside the tissue is shortened by the amount of the translation Δx, while an additional element of length Δx is added to the last element of the needle. Assuming that the last (n + 1) element is relatively small, and that the forces on it create negligible moments, its shape is taken as a straight line with the slope of the previous element.
In summary, given the translation and a rotation of the needle base, we are now able to calculate the 3-DOF translation and rotation of the needle tip, thus completing the forward kinematics solution.
Inverse kinematics
In an actual needle insertion problem, there is a need to hit the target with the tip while at the same time avoiding possible organ obstacles. Therefore, a particular trajectory is desired for the tip of the needle and it is the manipulation to be performed at the needle base that is calculated. This is an inverse kinematics problem, i.e., given the position and orientation of the tip trajectory, the translation and orientation of the needle base is derived.
Let us expand the matrix in equation (3):
Given the base translation Y and rotation θ, one can solve for Ni,j. Note that the last two elements of vector N are the translation and rotation of the tip. In the inverse kinematics problem, the translation and rotation of the tip − Nn3 and Nn4–are known and the unknowns are the translation and rotation of the base – Y and θ or N11 and N12. As in the last two equations the two variables are known, one can write equation (4) as:where
are the original vectors
without the last two elements. K̃21 is an (n − 2) × (n − 2) matrix, and equation (5) can be solved for
and therefore for Y and θ, which is the solution of the inverse kinematics.
Path planning
Planning of a linear insertion path is trivial. The main challenge is avoiding obstacles while applying minimal lateral pressure to the tissue. In the presence of obstacles, the optimal needle path requires minimal curvature of the needle. The path planning problem thus reduces to finding the shortest curve that connects the target to the needle insertion point, which avoids the obstacle while maintaining minimal needle curvature.
As every step is dependent on the history of the insertion, full simulation of the needle insertion is required. shows several solutions of the path planning where the target point is reached with different tip inclinations.
Figure 3. Several needle path solutions for the same tip position with different tip inclinations. [Color version available online.]
![Figure 3. Several needle path solutions for the same tip position with different tip inclinations. [Color version available online.]](/cms/asset/b492ea57-ab75-487f-8fb1-ab233b0cecd7/icsu_a_189223_f0003_b.jpg)
As the orientation of the tip is of less importance in biopsy, one can choose from the infinite solutions the one that applies minimal pressure to the tissue. This is achieved by minimizing the sum of squares of the virtual springs deflections, the sum of which is given by S:
Differentiating equation (6) with respect to θt and equal to zero one obtains:
Equation (7) is then substituted into equation (4) for of the slope of the last element Nn4.
Needle insertion simulation
Simulations of the needle insertion procedure were carried out. The motion of the needle base induces the needle tip to execute a sine wave path in order to avoid an obstacle in the tissue. In and , the left red circle represents an obstacle and the right one is the target.
Figure 4. Needle insertion simulation for tip orientation tangent to the path. [Color version available online.]
![Figure 4. Needle insertion simulation for tip orientation tangent to the path. [Color version available online.]](/cms/asset/f60ca8b1-14cc-491e-8645-a3ef76f0244d/icsu_a_189223_f0004_b.jpg)
Figure 5. Needle insertion simulation minimizing lateral pressure on the tissue. [Color version available online.]
![Figure 5. Needle insertion simulation minimizing lateral pressure on the tissue. [Color version available online.]](/cms/asset/85e2976a-90c5-4352-a3a5-d9ee9e399246/icsu_a_189223_f0005_b.jpg)
As the needle penetrates deeper into the tissue, additional virtual springs are introduced and are shown, for simplicity, as a compressible mesh below and above the needle. The needle trajectory is shown for two cases: one where the tip is forced to be tangent to the tip trajectory (), and another where the trajectory is optimized for minimal lateral tissue pressure (). The simulation starts with one element beam of length 100 mm at step 1. After advancement of 10 mm into the tissue, the element is subdivided, adding a new element of 10 mm and a virtual spring at the tip of the needle. This is repeated such that at the 6th step there are 6 elements and 6 virtual springs.
Results
Two metal pieces were inserted into animal tissue, one as the target and the other as the obstacle. A robot manipulating a needle was then programmed to follow a path, computed using the above algorithm, to avoid the obstacle and hit the target.
The experiment was conducted on chicken breast muscle and animal liver using the RSPR parallel robot Citation[16] shown in . The needle is connected to the robot through an ATI Nano17 6-DOF force sensor, and is a spinal 22G × 90 mm type with an outer diameter of 0.71 mm. With proper lighting it was possible to see through the tissue and to obtain images of reasonable quality. The tissue is placed in a 100 mm × 100 mm frame and is constrained in one plane between two glass plates. The stiffness coefficient of the virtual springs was evaluated experimentally to be 60 N/m by needle force measurement.
Figure 6. Needle insertion by robot corresponding to simulation steps 1, 3, 4 and 6 (see ). [Color version available online.]
![Figure 6. Needle insertion by robot corresponding to simulation steps 1, 3, 4 and 6 (see Figure 5). [Color version available online.]](/cms/asset/56a81af7-64ea-45f8-b33d-35898966e5ff/icsu_a_189223_f0006_b.jpg)
The images corresponding to steps 1, 3, 4 and 6 of the simulation described in the section above (see ) are shown in . As can be seen, the path followed by the needle tip was similar to the simulated path; the tip avoided the obstacle and hit the target.
Experiment with X-ray imaging
The experimental system is shown in ; it comprises a Siemens C-arm for imaging the RSPR 6-DOF parallel robot, a 6-DOF force sensor, and the spinal needle.
Figure 7. a) Experimental setup with X-ray imaging. b) Spinal needle, force sensor and liver tissue. [Color version available online.]
![Figure 7. a) Experimental setup with X-ray imaging. b) Spinal needle, force sensor and liver tissue. [Color version available online.]](/cms/asset/dd471c40-0f62-4650-9d6c-5c2301d92dfb/icsu_a_189223_f0007_b.jpg)
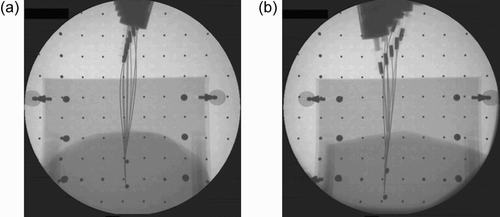
The needle insertion procedure is as follows. First, a single X-ray image of the needle and the tissue is taken (). The user then points to the target and the obstacles on the screen. The computer applies the needle detection algorithm described above to find the position of the needle tip and to calculate the needle insertion path to the target that avoids the obstacles. The detected needle and the planned needle path are then drawn on the image (). Next, the needle insertion path is simulated to check in advance for needle-tissue lateral forces and to confirm the robot's capability to execute the required trajectory. The system is then considered ready to carry out the needle insertion, as shown in and . It can be seen that the needle is bent so that it hits the target while the obstacle is avoided.
Figure 8. a) Needle and tissue image. b) Detected needle and planned insertion path. [Color version available online.]
![Figure 8. a) Needle and tissue image. b) Detected needle and planned insertion path. [Color version available online.]](/cms/asset/4c85c7cc-08d9-4b29-9cbc-0f2a076817f0/icsu_a_189223_f0008_b.jpg)
It should be noted that the obstacle and target metal balls appears in the images as ellipses because they move during the insertion.
Discussion
This investigation develops the planar linearized model approximation assuming small deflections to address the flexible needle insertion problem. Using this model, the inverse and forward kinematics of needle insertion are calculated in one step by solving a low-dimensional linear system of equations. Additionally, path planning and its optimization for minimal tissue pressure were developed. It was found that this model can be solved in a closed form for a given needle tip trajectory. Relaxing the requirement that tip orientation be tangent to the path at each point greatly decreases both the needle base stroke and the lateral pressure exerted on the tissue. Using a robot to maneuver the needle base and insert the needle into animal tissues verified the proposed concept. The needle trajectory was similar to the preplanned trajectory, avoiding an obstacle and hitting the target. Imaging the tissue by X-ray enabled an operator to mark on a screen the target and the obstacle from which a computer calculated the needle tip path and the corresponding needle base motion required to accomplish this tip path.
References
- DeAndres J, Reina M A, Lopez-Garcia A. Risks of regional anesthesia: Role of equipment – Needle design, catheters. Proceedings of the VII Annual European Society of Regional Anaesthesia Congress, Geneva, Switzerland, September, 1998
- DiMaio S P, Salcudean S E. Needle insertion modeling and simulation. IEEE Trans Robotics Automation October, 2003, Special issue on Medical Robotics
- Alterovitz R, Pouliot J, Taschereau R, Hsu I J, Goldberg K. Sensorless planning for medical needle insertion procedures. Proceedings of the 2003 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS 2003), Las Vegas, NV, October, 2003
- Grant A, Neuberger J. Guidelines on the use of liver biopsy in clinical practice. Gut 1999; 45(Suppl 4)IV1–IV11
- Chohan U, Hamdani G A. Post dural puncture headache. J Pak Med Assoc 2003; 53(8)359–367
- DiMaio S P, Salcudean S E (2003) Needle steering and model-based trajectory planning. Proceedings of the 6th International Conference on Medical Image Computing and Computer–Assisted Intervention (MICCAI 2003), MontrealCanada, November, 2003, R E Ellis, T M Peters. Springer, Berlin, 33–40, Part I. Lecture Notes in Computer Science Vol 2878
- Mineta T, Mitsui T, Watanabe Y, Kobayashi S, Haga Y, Esashi M. Batch fabricated flat meandering shape memory alloy actuator for active catheter. Sensors and Actuators A: Physical 2001; 88: 112–120
- Kataoka H, Washio T, Audette M, Mizuhara K (2001) A model for relations between needle deflection, force, and thickness on needle penetration. Proceedings of the 4th International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI 2001), UtrechtThe Netherlands, October, 2001, W J Niessen, M A Viergever. Springer, Berlin, 966–974, Lecture Notes in Computer Science Vol 2208
- O'Leary M, Simone C, Washio T, Yoshinaka K, Okamura A. Robotic needle insertion: Effects of friction and needle geometry. Proceedings of the IEEE International Conference on Robotics and Automation, 2003 (ICRA '03), Taipei, Taiwan, September, 2003; 1774–1780
- Ebrahimi R, Okzawa S, Rohling R, Salcudean S (2003) Hand-held steerable needle device. Proceedings of the 6th International Conference on Medical Image Computing and Computer–Assisted Intervention (MICCAI 2003), MontrealCanada, November, 2003, R E Ellis, T M Peters. Springer, Berlin, 223–230, Part II. Lecture Notes in Computer Science Vol 2879
- Park W, Kim J S, Cowan Y Z, Okamura A M, Chirikjian G S. Diffusion–based motion planning for a nonholonomic flexible needle model. Proceedings of the 2005 IEEE International Conference on Robotics and Automation, BarcelonaSpain, April, 2005
- Alterovitz R, Goldberg K. Planning for steerable bevel–tip needle insertion through 2D soft tissue with obstacles. Proceedings of the 2005 IEEE International Conference on Robotics and Automation, BarcelonaSpain, April, 2005
- Webster R J, III, Memisevic J, Okamura A M. Design considerations for robotic needle steering. Proceedings of the 2005 IEEE International Conference on Robotics and Automation, BarcelonaSpain, April, 2005
- Simone C, Okamura A. Haptic modeling of needle insertion for robot-assisted percutaneous therapy. Proceedings of the IEEE International Conference on Robotics and Automation, 2002 (ICRA '02), Washington, DC, May, 2002; 2085–2091
- Fung Y C. Biomechanics: Mechanics Properties of Living tissues. 2nd edition. Springer, New York 1993; 277
- Simaan N, Glozman D, Shoham M (1998) Design considerations of new six degrees-of-freedom parallel robots. Proceedings of the IEEE International Conference on Robotics and Automation, LeuvenBelgium, May, 1998. 2: 1327–1333