Abstract
For a fetus diagnosed with a severe congenital anomaly, surgery may offer an alternative to abortion, intra-uterine death, or a life with disability. Expertise is limited however, to a few treatment centers worldwide, and there are many technical hurdles to overcome, including requirements for miniaturized instrumentation, real-time high-resolution imaging, and harmless fetal access. This article highlights recent practices in prenatal intervention and various initiatives to integrate robotics into the fetal operating room. While the number of potential patients is low, research for implementation of robotics in the field of fetal surgery is justified by the morbidity rates of current procedures, proven favorable outcomes with intervention, and the educational value with potential for extension to other medical disciplines.
Introduction
Fetal surgery is a medical discipline that is still in its formative stages. It draws on expertise from an assortment of clinical specialties to enable prenatal intervention to correct congenital defects. Michael Harrison, the father of fetal surgery, performed the first successful human fetal surgical procedure in 1983 at the University of California, San Francisco, (UCSF) when he corrected a urinary tract obstruction. This breakthrough encouraged Harrison and colleagues to address other anomalies that would lead to fetal demise without prenatal treatment, including congenital cystic adenomatoid malformation (CCAM), congenital diaphragmatic hernia (CDH), sacrococcygeal teratoma (SCT), and monochorionic twin syndromes such as twin-to-twin transfusion syndrome (TTTS) and twin reverse arterial perfusion (TRAP). Additional comprehensive centers for fetal therapy have emerged worldwide and should lead to improved competence in human fetal surgery, though rapid development in this field has been hindered by low patient volume, a steep learning curve, and the paucity of dedicated instrumentation Citation[1].
This article proceeds with a general description of the diagnosis and surgical treatment of congenital fetal syndromes. Robotic applications in fetal surgery are then introduced, followed by a review of technical challenges specific to minimally invasive fetal surgery and a description of a micro-robotic alternative suggested by a leading fetal surgeon. Lastly, a micro-robot concept for treatment of CDH is proposed.
Clinical intervention
The congenital syndromes most commonly operated upon in utero may be diagnosed as early as the 10th week of gestation by means of fetal imaging modalities such as 2D/3D ultrasound and ultrafast MRI. Clinical intervention to correct these malformations might eliminate undesirable vasculature with radiofrequency ablation or Nd:YAG laser, deploy a shunt or balloon by means of a catheter, or protectively enclose a protruding spinal cord.
Historically, surgeons initially accessed the fetus by performing a hysterotomy, similar to the surgical approach in a Caesarean section. A trans-abdominal incision was made in the uterus, then the part of the fetus to be operated on was extracted, surgically corrected, and replaced inside the uterine sac, which was subsequently closed with sutures. Severe post-operative complications related to the large uterine incision included premature rupture of membranes (PROM) and pre-term labor (PTL), which may be associated with significant fetal (and maternal) mortality and morbidity, including developmental delay and disability Citation[2].
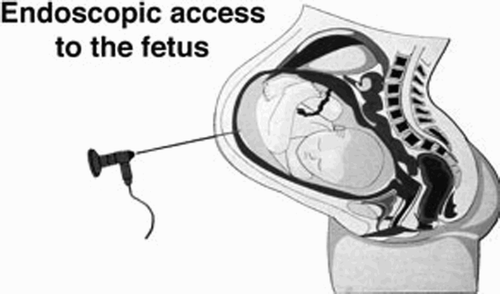
Fetal surgeons devised a less invasive approach that is referred to as operative fetoscopy. In this approach, surgical tools are inserted into the uterus through multiple ports under ultrasonic and endoscopic guidance, as illustrated in . Instrumentation in a fully percutaneous access system penetrates the maternal abdominal wall, often with fewer than three entry sites, each less than 5 mm in diameter. Fetoscopy is clearly preferable to open surgery as it minimizes fetal stress and manipulation by operating on the fetus in its natural environment. It also reduces uterine trauma, specific to single-trocar procedures, thereby helping to prevent PROM and PTL. Furthermore, incisions of less than 2-3 mm are less apt to leak, presumably closing under myometrial contraction Citation[3].
Figure 1. Minimally invasive access to the fetus. (Reprinted with permission from M. Harrison, http://www.fetus.ucsf.edu/)
Integration of robots
Robots can provide various technical advantages in fetal surgery. They assist in manipulation of delicate fetal structures by simultaneously magnifying the surgical field, filtering hand-tremor, scaling up subtle forces and introducing haptic feedback to endoscopic tools. Master–slave systems enable access to limited surgical expertise via telesurgery. To date, three institutions have performed minimally invasive robotic fetal surgery with pregnant ewes (), and two human cases of instructive telesurgery have been reported.
Table I. Summary of robotic fetal surgeries on pregnant ewes.
Tulipan and colleagues at Vanderbilt University were historically more successful with open intervention than with fetoscopy in treating myelomeningocele (MMC), a form of spina bifida aperta. They nonetheless revisited the minimally invasive approach with the da Vinci system (Intuitive Surgical, Mountain View, CA) to establish the potential of robotic fetal surgery Citation[4]. Percutaneous access was initially prohibited due to lack of experience. Instead, a 4–8 cm incision through the abdomen, referred to as laparotomy, exposed the intact external uterine surface. Three expandable sleeves inserted through the uterine wall established operative ports to host the robotic instruments. A 5-mm 30° laparosope (Karl Storz, Tuttlingen, Germany) and integration of images from dual cameras generated a 3D view of the surgical field.
The fetal lambs operated upon in the Vanderbilt study were at the equivalent age of 20–24 weeks of human gestation. Once the robot arms were inside the uterus, operative time was less than 3 hours following a dramatic experiential learning curve. Unfortunately, two lambs died intraoperatively, ostensibly as the result of umbilical cord entanglement inducing hypoxia. The precision obtained using the robot was a clear improvement over that with the manual procedure, though a fetal scalp monitor would have enabled better patient observation throughout the procedure. This group experimented with a further three lambs in pursuit of their fully percutaneous goal, triumphantly in one case. Time constraints have prevented further experiments, though they still foresee a future for robotic intervention (N.B. Tulipan – personal communication).
Again using pregnant ewes as the experimental subjects, the team of Kohl et al. at the University of Bonn performed several successful fully percutaneous patch coverages of lumbosacral skin lesions characteristic of spina bifida, using the Zeus robot system (Computer Motion, Santa Barbara, CA) Citation[5]. They conducted three manual procedures using 4-mm trocars as a control group, and attempted four robotic closures with larger trocars for the robotic arms.
Kohl et al. determined that an anteriorly located placenta is essential for percutaneous access to the fetus for multi-trocar procedures, though more recently he has discovered techniques to address this handicap. Furthermore, the lumbrosacral region should be postured directly under the operative robot arms. During experimentation, one ewe exhibited uterine membrane separation, and an alternative entry site was selected in order to continue the surgery. The robot arms were reoriented to accommodate the new entry site, but this configuration induced unsynchronized robotic arm motion. As a result, this ewe did not complete the percutaneous experiment. The remaining three ewes underwent successful simulation and repair of an MMC lesion with a 150–300 mm2 collagen or Goretex patch. Removal of the 4 mm trocars in the control group did not result in leakage, whereas insertion sites for the 8-mm trocars associated with the robot required additional closure. Robotic MMC repair was completed in a timeframe similar to that reported by Tulipan, but longer in comparison with that for the manual procedure. Although robot setup was time-consuming, the robotic assistance added precision and reduced physical strain for the surgeon. Kohl concluded that, for simple coverage of MMC, the robot offered no advantage over the manual approach, though implementation of surgical robotics would be important if more complex repairs were required.
Knight and colleagues at Wayne State University and Children's Hospital of Michigan implemented a comprehensive 26-ewe trial with a dedicated Zeus robot Citation[6]. In this case, the uterus was anchored to the maternal abdominal wall to assist trocar insertion and reduce membrane separation and leakage. Fetal position dictated the selection of simulated malformation: either an abdominal hernia if the fetus was oriented dorsum toward the surgical field, or MMC in fetuses with ventral-anterior posture.
This group witnessed unexpected robot arm movement, as well as severe insufflation leaks with open fetal surgery. In the laparotomy approach, they found that the flaccid uterine wall inadequately supported the forces that the robot arms imposed on the trocar. In contrast, the trocar in the percutaneous technique was amply supported by layers of abdominal tissue. Percutaneous fetal surgery was thus considered the most promising of the three methods when using the Zeus robot. This group has speculated that as the da Vinci design does not rely on the trocars as a fulcrum point, it will perform better than Zeus during a laparotomy approach.
Membrane separation between the amnion and chorion was more acute in the percutaneous cases, and one of the three affected fetal ewes did not survive. Other obstacles common to fetal surgery included uterine access and fetal positioning. Once the uterus barrier was penetrated and the fetus held in place, the procedures progressed smoothly. The robot added dexterity to suture manipulation and the procedure was completed in 30 min without complication. Both Knight and Kohl foresee primate models as the next stage in experimentation.
Two minimally invasive fetal surgeries, conducted in Santiago, Chile, and Brisbane, Australia, were guided by video telecommunication from Tampa, Florida. These telesurgeries did not involve the use of robots, but established the viability of remote master–slave fetal procedures. In the first case, Tampa-based surgeons coached an acardiac twin reduction in order to resolve TTTS Citation[7]. Video images captured in the Chilean operating theater were transmitted via ISDN. In contrast, the live broadcast between Tampa and Brisbane used a dedicated Internet link to transmit video images. The Internet Protocol connection maintained image quality equal to that of ISDN, but demanded greater technical coordination Citation[8].
Complications with minimally invasive fetal therapy
While minimally invasive surgery has positively affected fetal therapy, it also has several disadvantages. In a percutaneous procedure, the fetoscopic tool must traverse three boundaries: the mother's abdomen, the uterus, and a mobile fetus. The rigid instrument is constrained circumferentially between the first two boundaries, limiting the majority of motion to the direction along the length of the tool. Once inside the uterus, the tool must interact with the fetus, which is able to move freely in all directions. This is a mechanically difficult feat for a rigid device. Moreover, use of any tool larger than 2–3 mm increases the risk of pre-term labor Citation[1]. Repeated tool insertion into and out of the trocar induces uterine tenting, and separation and shredding of the chorion and amnion membranes.
Fetal imaging in a minimally invasive surgical approach is yet another source of complexity. In the first place, a sufficient intra-uterine acoustic window is necessary to produce a high-quality ultrasound scan. Availability of such a window is limited in some congenital syndromes characterized by oligohydramnios or low quantities of amniotic fluid. Next, images from a fetoscopic camera may be blurry due to turbidity of the amniotic fluid, particularly in advanced stages of pregnancy. To improve clarity, surgeons remove small quantities of amniotic fluid and insufflate the uterus with CO2 or N2O. The latent effect of these gases on human fetal health is as yet indeterminate. Finally, a lack of intravenous access to the fetus results in suboptimal monitoring.
The micro-robot: an alternative solution
A micro-robot that is inserted into the uterus and is capable of actuation may offer a more benign alternative to fetoscopic surgery Citation[1]. The surgeon could remotely guide the micro-robot to various positions with respect to the fetus, and the device could be equipped with imaging and interventional capabilities. For instance, it could grind a well-defined CCAM tumor or walk along the surface of vessels, delicately ablating undesired vasculature. It could also clear a urinary tract obstruction or establish a conduit from the fetal bladder to the amniotic fluid. Intervention for MMC and abdominal wall defects could initially provide images and monitor fetal condition, while a more sophisticated micro-manipulator could eventually repair the defects.
A micro-robot could replace the needles currently used in amniocentesis, cordocentesis (fetal blood sampling from the umbilical cord), and fetal drug delivery. Micro-robotic technology also holds the promise of facilitating future advances in stem cell and genetic therapy by conveying site-specific injections Citation[1]. Once safety and performance have been demonstrated, use of the micro-robot could be expanded to correct less severe fetal anomalies, and it could be adapted for other surgical disciplines.
Current micro-robots inside the human body
To the best of the authors' knowledge, no current research fuses micro-robotics and surgical intervention inside the uterus. NASA collaborated with UCSF in the early 1990s to build an implantable monitoring device transmitting fetal heart rate, temperature and intra-uterine pressure via radiotelemetry. The 25mm-diameter capsule was experimentally inserted into the abdomen of a sheep. Miniaturization initiatives for testing on the International Space Station have been deferred due to budget constraints (J. Hines, NASA Manager of Biomolecular Sensor Development – personal communication). Three academic groups have begun to investigate fetal surgery instrumentation. Projects include a device to measure fetal cardiac tissue properties Citation[9], a motor-driven fetoscopic end-effector guided by open MRI Citation[10], and an electromagnetic tool navigation unit integrated with 3D ultrasound (Children's Hospital of Boston, www.crisp.cit.nih.gov). Meanwhile, the Karl Storz company is designing an instrumentation kit specifically for fetoscopy.
Only a few institutions worldwide have considered self-propelled micro-devices for medical application, predominantly designs intended to use inchworm-like locomotion in the GI tract. Research concentration in this area can be attributed to the relatively large diameter of the intestine and the non-sterile environment, which is less sensitive to foreign materials. The 13mm-diameter tethered inchworm developed at the University of Pisa boasts a 3-degree-of-freedom pneumatically controlled steering system and an onboard camera, and is externally powered (A. Menciassi, April 2004 – personal communication). A 19mm-diameter tethered robot designed by a group at Carnegie Mellon University (CMU) (Pittsburgh, PA) traveled 10 mm/second with an onboard CCD camera Citation[11]. Unfortunately, the robot became entrapped in the intestinal folds and was challenged by the changing inner wall diameter, while the unpredictable surrounding terrain caused navigation problems. A more recent effort at CMU advocates the use of micro-fibers to mimic van der Waal adhesion forces characteristic of gecko cilia motion Citation[12]. Similarly, Edd et al. Citation[13] suggested the use of carbon nanotubes to manufacture such cilia. Kosa et al. Citation[14] have described a swimming modality in which a traveling wave is passed along the length of an elastic tail in order to propel a micro-robot through the cerebrospinal fluid. Finally, Stereotaxis (St. Louis, MO), Ishiyama et al. Citation[15], Guo et al. Citation[16] and Yesin et al. Citation[17] have all engaged magnetic fields to steer their magneto-sensitive devices through the body.
MRL Febot
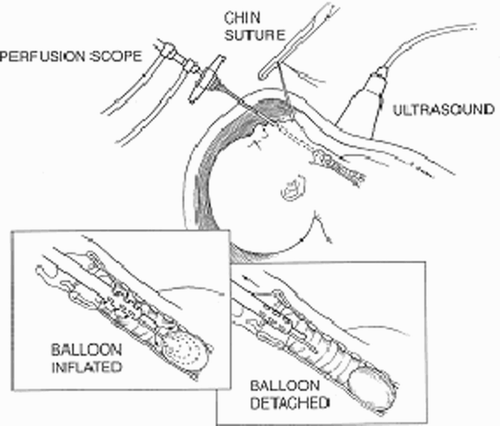
The Medical Robotics Laboratory (MRL) at the Technion proposes to build a fetal micro-robot with tracheal occlusion for CDH planned as its first surgical intervention. CDH is a perforation in the diaphragm muscle through which abdominal viscera protrude into the chest cavity, compressing the lung and interfering with its proper development. Experimentation led to an approach that reverses the pathophysiology by temporarily occluding the trachea at 24-28 weeks gestation. Tracheal occlusion (TO) obstructs the egress of fluid formed by the fetal lung in order to maintain airway patency and lung growth Citation[18]. A detachable silicon balloon deployed inside the trachea is administered with a 3 mm bronchoscope via a single portal, as shown in . The balloon can either be recovered during gestation or upon delivery. The micro-robot will be surgically inserted into the uterus, navigate the uterus through the amniotic fluid, climb along the inner surface of the trachea, and deploy a balloon to temporarily obstruct the pathway of the lung fluid.
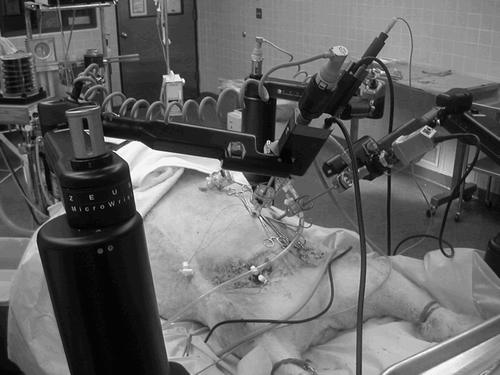
Figure 2. Setup of Zeus-assisted surgery. (Contributed by C. Knight.) (Color version available online.)
An onboard CMOS camera improves upon current ultrasound imaging and may result in better visualization of intra-uterine contents than with a rigid fetoscope. The robot can either swim through the amniotic fluid or gently crawl along the intra-uterine and fetal tissues. The diametric dimension is limited to 2 mm to minimize the risk of pre-term labor, whereas the lengthwise dimension is flexible. The robot design may include polymer actuators, which operate in an ionic fluid environment such as that provided by amniotic fluid. Locomotion may be achieved by means of gaseous bubbles generated inside the robot by a chemical interaction of two compounds. Once the bubbles are expelled from the micro-robot into the surrounding fluid, they gradually expand and ultimately burst. A continuous stream of bubbles emanating from a rear outlet in the micro-robot results in net forward displacement and thus self-actuation.
The motivation for this effort is to improve fetal therapy by introducing a micro-sized robot into the uterus in order to correct or reverse the effects of congenital anomalies using less invasive techniques. However, the pre-eminent consideration for any fetal robot design will be to ensure maternal and fetal safety.
Conclusion
For fetuses diagnosed with a number of severe syndromes, surgery offers an alternative to abortion, intra-uterine death or a life with disability. While the number of potential patients is statistically low, fetal robot research is justified by the high morbidity rates associated with current techniques and the educational value with potential for extension to other surgical procedures. Favorable outcomes have been demonstrated with fetal surgical intervention, despite existing complications. A micro-robot has the potential to eliminate or resolve these complications and thus further advance fetal care. We hope that this article will stimulate an academic interest in fetal robotics, a synergy of medicine and technology that will continue to both build upon and extend the cutting edge in both fields.
Acknowledgments
The authors thank Drs. Alan Flake, Colin Knight, Thomas Kohl and Noel Tulipan for their clinical contributions to this paper, as well as Drs. Megan Moore and Michael Klompas for their writing expertise.
References
- Flake A W. Surgery in the human fetus: the future. J Physiol 2003; 547(Part 1)45–51
- Sydorak R M, Albanese C T. Minimal access techniques for fetal surgery. World J Surg 2003; 27(1)95–102
- Gratacos E, Wu J, Yesildaglar N, Devlieger R, Pijnenborg R, Deprest J A. Successful sealing of fetoscopic access sites with collagen plugs in the rabbit model. Am J Obstet Gynecol 2000; 182(1 Part 1)142–146
- Aaronson O S, Tulipan N B, Cywes R, Sundell H W, Davis G H, Bruner J P, Richards W O. Robot-assisted endoscopic intrauterine myelomeningocele repair: a feasibility study. Pediatr Neurosurg 2002; 36(2)85–89
- Kohl T, Hartlage M G, Kiehitz D, Westphal M, Buller T, Achenbach S, Aryee S, Gembruch U, Brentrup A. Percutaneous fetoscopic patch coverage of experimental lumbosacral full-thickness skin lesions in sheep. Surg Endosc 2003; 17(8)1218–1223
- Knight C G, Lorincz A, Johnson A, Gidell K, Rabah R, Klein M D, Langenburg S E. Robot-enhanced fetoscopic surgery. J Pediatr Surg 2004; 39(10)1463–1465
- Quintero R A, Munoz H, Pommer R, Diaz C, Bornick P W, Allen M H. Operative fetoscopy via telesurgery. Ultrasound Obstet Gynecol 2002; 20(4)390–391
- Chan F Y, Soong B, Taylor A, Bornick P, Allen M, Cincotta R, Quintero R. Fetal endoscopic telesurgery using an Internet Protocol connection: clinical and technical challenges. J Telemed Telecare 2003; 9(Suppl 2)S12–S14
- Eisinberg A, Tonet O, Dario P, Macri G, Carrozza M C. Microfabricated instrument for haptic tissue recognition in fetal cardiac surgery. Proceedings of the First IEEE/RAS-EMBS International Conference on Biomedical Robots and Biomechatronics (BioRob 2006), PisaItaly, February, 2006, 1183–1188
- Harada K, Tsubouchi K, Fujie M G. Micro manipulators for intrauterine fetal surgery in open MRI. In:. Proceedings of International Conference on Robotics and Automation (ICRA 2005), BarcelonaSpain, April, 2005, 504–509
- Asari V K, Kumar S, Kassim I M. A fully autonomous microbotic endoscopy system. Journal of Intelligent and Robotic Systems 2000; 28: 325–341
- Karagozler M E, Cheung E, Kwon J, Sitti M. Miniature endoscopic capsule robot using biomimetic micro-patterned adhesives. In:. Proceedings of the First IEEE/RAS-EMBS International Conference on Biomedical Robots and Biomechatronics (BioRob 2006), PisaItaly, February, 2006, 105–111
- Edd J, Payen S, Rubinsky B, Stoller M L, Sitti M. Biomimetic propulsion for a swimming surgical micro-robot. IEEE Intelligent Robotics and Systems 2003; 3: 2583–2588
- Kosa G, Shoham M, Zaaroor M. Analysis of swimming micro robot. Proceedings of the First IEEE/RAS-EMBS International Conference on Biomedical Robots and Biomechatronics (BioRob 2006), PisaItaly, February, 2006, 131–135
- Ishiyama K, Arai K I, Sendoh M, Yamazaki A. Spiral-type micro-machine for medical applications. Proceedings of the International Symposium on Micromechatronics and Human Science, NagoyaJapan, October, 2000, 65–69
- Guo S, Fukuda T, Asaka K. A new type of fish-like underwater microrobot. IEEE Trans Mechatronics 2003; 8(1)136–141
- Yesin K B, Vollmers K, Nelson B J. Analysis and design of wireless magnetically guided microrobots in body fluids. Proceedings of the International Symposium on Robotics and Automation (ISRA 2004), QueretaroMexico, August, 2004, 1333–1338
- Harrison M R, Keller R L, Hawgood S B, Kitterman J A, Sandberg P L, Farmer D L, Lee H, Filly R A, Farrell J A, Albanese C T. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Engl J Med 2003; 349(20)1916–1924