Abstract
Objective: One of the difficult steps in intra-medullary nailing of femoral shaft fractures is distal locking – the insertion of distal interlocking screws. Conventionally, this is performed using repeated image acquisitions, which leads to considerable irradiation of the patient and surgical team. Virtual fluoroscopy has been used to reduce radiation exposure, but can only provide multi-planar two-dimensional projection views. In this study, two calibrated fluoroscopic images were used to automatically recover the positions and orientations of the distal locking holes (DLHs). The ultimate goal is to provide precise three-dimensional guidance during distal locking.
Methods: A model-based optimal fitting process was used to reconstruct the positions and orientations of the DLHs from two calibrated fluoroscopic images. No human intervention is required. A preliminary in vitro validation study was conducted to analyze the accuracy and reliability of the technique using images acquired from different viewpoints. The ground truths of the DLH were obtained by inserting a custom-made steel rod through the hole and then digitizing both the top and bottom centers of the rod using a sharp pointer. The recovery errors were computed by comparing the computed results to the ground truths.
Results: In all experiments, the poses of the DLHs could be recovered fully automatically. When the recovered positions and orientations of the DLHs were compared to their associated ground truths, a mean angular error of 0.5° (STD = 0.2°), and a mean translational error of 0.1 mm (STD = 0.0 mm) were found.
Conclusions: Accurate and reliable pose recovery of distal locking holes from two calibrated fluoroscopic images is achievable. Our preliminary in vitro experimental results demonstrate that the recovered poses of the distal locking holes are sufficiently accurate for intra-operative use.
Introduction
Femoral shaft fractures are one of the most common injuries encountered in routine trauma surgery. Most of them are displaced and need to be surgically reduced. Intra-medullary nailing with reaming is an excellent operative procedure that has revolutionized the treatment of fractures of the femoral shaft. The efficacy of statically locked intra-medullary nails inserted after reaming in the treatment of fractures of the femoral shaft has been well established Citation[1–3]. The surgical procedure involves the fixation of long-bone fractures by inserting an intra-medullary nail (IMN), which is a long, prefabricated, locking rod, into the medullary canal of the damaged bone. The surgeon introduces the implant by making an incision in the proximal femur, reaming the medullary canal, reducing the bone fragments, inserting the IMN through the fragments, and finally locking the IMN with screws to create a means of internal support. The transverse interlocking screws inserted into the proximal and distal holes of the IMN are essential for controlling the rotation and translation of the bone fragments with respect to one another. In comminuted fractures, these interlocking screws also bear the transmitted load until the fracture has consolidated Citation[3]. To insert the transverse interlocking screws, it is necessary to align and drill through the bone to meet the proximal and distal interlocking screw openings of the IMN.
Conventionally, the proximal locking is relatively easy since a mechanical jig attached to the proximal end of the nail allows insertion of the proximal screws with sufficient precision. The difficult part of intra-medullary nailing of femoral shaft fractures is the insertion of the distal interlocking screws. Complicating the process of locating and inserting the distal interlocking screw is the deformation of the nail during insertion. It has been reported that deformation occurs in several planes due to medial-lateral (ML) and anterior–posterior (AP) flexion of the distal nail after it has been inserted Citation[4]. Using a magnetic tracking system in a cadaveric study, Krettek et al. Citation[4] reported the following deformation measurement results for small-diameter nails and large-diameter nails, respectively: lateral translations of 18.1 ± 10.0 mm and 21.5 ± 7.9 mm; dorsal translations of −3.1 ± 4.3 mm and 0.4 ± 9.8 mm; and rotation about the longitudinal axes of −0.1 ± 0.2° and 10.0 ± 3.1°. The reason for the wide variations in insertion-related femoral nail deformation is the fact that the nail has to deform to the shape of the medullary canal upon insertion. The shape of the canal varies widely from person to person, and it is not possible to predict how the nail will deform. It is therefore very difficult to determine the resultant locations and orientations of the DLHs relative to their initial positions. In a conventional surgical procedure, the surgeon depends heavily on intra-operative X-rays to provide precise locations and orientations of the DLHs. This requires positioning the axis of the fluoroscope perpendicular to the locking holes so that the holes appear perfectly circular in the images. This is achieved through a trial-and-error method and requires prolonged X-ray exposure for both the surgeon and patient. The duration of the surgeon's direct exposure to radiation for each conventional procedure has been reported as 3 to 30 min, with approximately 30–50% of this exposure occurring during distal locking Citation[5].
Virtual fluoroscopy has been used to reduce radiation exposure, but can only provide multi-planar two-dimensional (2D) projection views of the nail Citation[6], Citation[7]. The desire to target accurately with the minimum X-ray exposure has led to various attempts to develop image-based methods for recovering the positions and orientations of DLHs. Methods based on bi-planar fluoroscopic images Citation[8–11] or on a single image Citation[12] have been presented previously. However, the existing methods suffer from certain disadvantages: The method described in references Citation[8–11] requires intraoperative user interactions to identify nail and hole projections from both images before applying an automatic process to find the poses of the DLHs. Two single-image-based methods are presented in reference Citation[12], but the more accurate one requires an image in which the holes appear to be perfectly circular, which normally requires the X-ray technician to use a try-and-move method several times.
This paper presents a preliminary in vitro study of a fully automatic approach to solving this problem based on two calibrated and registered fluoroscopic images. We opted for a two-image solution, because two images provide more information than a single image, especially for robust localization of the nail from fluoroscopic images with complex backgrounds. Using two images, we do not require an ML image with perfectly circular holes, which can only be achieved through a trial-and-error method involving more X-ray exposure.
Methods
Image calibration
In practice, the proximal fragment, the distal fragment and the nail may be treated as three rigid bodies and registered independently. The rigid transformations between these three rigid bodies can be trivially obtained from a navigator such as an optoelectronic tracker, a magnetic tracker, or even a medical robot Citation[13]. As this is not the focus of this paper, we here assume that the fractured femur has already been reduced and that the proximal and distal fragments are kept fixed relative to one another at the time of image acquisition. We also assume that the nail has been inserted to the distal end of the femur and locked proximally by a screw so that the complete femur and the nail can be treated as a single rigid body. A local coordinate system (COS) is established on this rigid body through a so-called dynamic reference base (DRB) technique Citation[14]. In the following description, let us denote this patient coordinate system as P-COS. Two fluoroscopic images S = {Sk, k = 1, 2} are then acquired; one from approximately the medial-lateral (ML) direction and the other from the anterior–posterior (AP) direction. Let us further denote the reference coordinate system in each C-arm shot Sk as A-COSk. The transformations Tk between P-COS and A-COSk at the time of acquisition of each fluoroscopic image can be obtained and recorded, and used to co-register the two independent fluoroscopic images. All computations now can be done in one world coordinate system, P-COS.
To relate a pixel in the 2D projection image Sk to its own reference coordinate system A-COSk, the acquired images have to be calibrated for physical projection properties and corrected for various types of distortion. In a previous paper from our institution Citation[15], a weak-perspective pinhole camera model was chosen for modeling the C-arm projection, as shown in . Weak-perspective is a good approximation when the depth range of the calibration object in the scene is small compared with the viewing distance, which is exactly the case in our application. Using such a camera model, a 2D pixel VI is related to a 3D point VA by the following equations Citation[15]:where ‖·‖ means calculation of the length of a vector, and the vectors fA, rA, cA and pI represent the position of the focal point, the vector along the image row increasing direction, the vector along the image column increasing direction, and the 2D position of the piercing point, respectively. They are projection parameters used to describe the projection properties of the C-arm and need to be calibrated pre-operatively.
Figure 1. Weak-perspective cone beam projection model is used for C-arm calibration. The X-ray is emitted at location fA and projects a spatial point vA onto the image plane as vI. pI is defined by the point where rays pass directly normal through the plane.
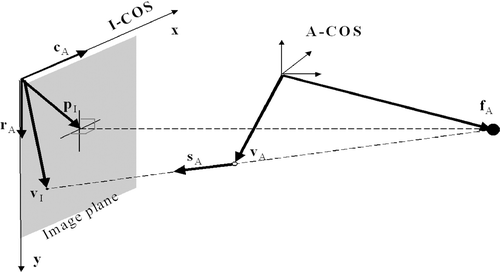
Equations (1) and (2) can be used for both forward and backward projections. For example, if we wish to calculate the direction SA of the backward projection ray of an image point VI, an additional constraint |SA|=1 can be used together with equation (2) to solve for this. The backward projection ray of point VI is then defined by the focal point fA and the direction SA.
Distortions arising from the pincushion effect (curved input screen) and the earth's magnetic field variations (s-shaped distortion) are different for each projection direction and hence must be corrected for. For this purpose, a calibration grid plate consisting of equally spaced fiducial markers with known positions was designed. This plate is mounted in front of the image intensifier. For each acquired image, the real projection of these fiducial markers is detected using image processing techniques, and their associated virtual projections are calculated based onequations (1) and (2). For each fiducial marker, a displacement vector that points from the real projection to the virtual projection is calculated. A bi-linear local interpolation is then performed to compensate for the distortion of each image pixel. Finally, the projections of these fiducial markers are removed from the undistorted images. For more details on these image-processing techniques and how to correct the distortions, see the earlier paper Citation[15].
Geometrical model
The distal part of the intra-medullary nail (DP-IMN) containing two DLHs – the focus of our interest – is modeled as a cylinder (, left) of known radius r. The geometrical model of the DLH is shown in the right portion of . It is a combination of an inner cylinder model and two cone trapezoid openings. For the purpose of simulating X-ray projections, the geometrical model of the DLH is described by four closed curves: two inner circles and two outer curves (, right). Each curve is approximated by a set of discrete points (visualized as red dots). Any position in between these points is calculated using linear interpolation.
Figure 2. Geometric model of the distal part of the nail (left; a cylinder with two distal locking holes) and the model of the distal locking holes (right; an inner cylinder model with two cone trapezoid openings). The model of the DLH is described by four closed curves: two inner circles and two outer curves. [Color version available online.]
![Figure 2. Geometric model of the distal part of the nail (left; a cylinder with two distal locking holes) and the model of the distal locking holes (right; an inner cylinder model with two cone trapezoid openings). The model of the DLH is described by four closed curves: two inner circles and two outer curves. [Color version available online.]](/cms/asset/2dbae198-391c-4cd0-b1ac-c5a103317b71/icsu_a_238697_f0002_b.gif)
To obtain the coordinates of those points used to approximate the model of the DLH, a local COS C′uvw is established by taking the intersection point C (also referred to as the center of the DLH) between the axis of the DLH and the axis of the DP-IMN as the origin, the axis of the DP-IMN as the u-axis, and the axis of the DLH as the v-axis (see for details). The coordinates of those points expressed in this local COS can be directly measured from a nail sample using a caliper, thanks to the symmetrical property of the DLH, or extracted from engineering drawings of the nail, if these are available.
Automatic image feature extraction
Observing the significant intensity difference between the nail projections and the bone projections, we apply a Canny edge detector with hysteresis Citation[16] to all the fluoroscopic images to automatically extract the “raw” edge pixels. The “raw” edge data are a combination of edges detected from the projections of the DP-IMN and the DLHs and false edges from image noise and projections of external objects such as patient reference fixation devices. We denote Ek as the set of all Nk detected 2D edge pixels from image Sk. For each edge pixel
, we always know its backward projection ray
using the method described in the Image calibration section above.
Automatic nail localization
The nail is localized by fitting the geometrical model of the DP-IMN (a cylinder of known radius) into both images. An iterative best-matched projection point (IBMPP) algorithm Citation[17], which is an extension of the algorithm presented in reference Citation[18] but with enhanced features, is combined with a random sample consensus (RANSAC) paradigm Citation[19] to effectively and robustly solve the fitting problem as follows:
Initialization. For image Sk, we first calculate the major axis AEk of the edge point set Ek by applying principal component analysis (PCA) to all edge pixels in Ek. The back-projection plane of the major axis AEk in P-COS is called the principal plane of image Sk. The initial axis of the DP-IMN is then estimated by computing the intersection line segment between two principal planes, as shown in .
Iterations. Starting from the initial position, the pose of the DP-IMN is iteratively estimated by fitting its geometrical model to both images using the following five-step algorithm:
Simulating X-ray projection: In this step, we simulate the X-ray projections of the geometrical model of the DP-IMN to each image, which are actually two line segments resulting from the silhouette projections of a cylinder model. If we discretize these two line segments, then for each 2D point on the segments we can calculate the associated 3D point on the surface of the cylinder model using the parametric equation of a cylinder.
2D matching: Observing that the virtual X-ray projections of the cylinder model are line segments, we design a shape-context-based operator to filter the detected edge pixels so that only those edge pixels whose neighboring edge pixels contain a line segment are kept. Let us denote these edge pixels as the valid edge pixels. Then, for each valid edge pixel, we try to find the closest point on the virtual X-ray projections of the cylinder model to set up a 2D matched point pair
, where
is one of the valid edge pixels and
is a model projection point whose associated 3D point Ci on the surface of the cylinder model is known.
Establishing 3D-2D correspondence: For each 2D matched point pair
, we can always find a 3D matched point pair
where point
is the closest point on the backward projection ray of
to point Ci.
Pose estimation: A paired-point matching algorithm Citation[20] is then applied to all calculated 3D matched point pairs to estimate a rigid transformation.
Pose upating: The pose of the geometrical model of the DP-IMN is updated by the computed rigid transformation.
Figure 3. Schematic view of the initialization for automatic nail localization. The thick dotted curves represent the detected edge pixel sets E1 and E2, including true edge pixels from nail projections and false edges from outliers; the thin dashed lines represent the major axis AEk computed by applying Principal Component Analysis (PCA) on Ek; O1 and O2 are the source points of C-arm shots S1 and S2, respectively.
These five steps are repeated until all pose parameters are converged.
3. Applying the random sample consensus paradigm. Not all valid edge pixels are helpful in nail localization: some of them are from the projections of outliers. This is handled by the RANSAC paradigm, which is an algorithm for robust fitting of the model in the presence of data outliers. Each time, a certain number of edge pixels (e.g., 10) are randomly sampled from those valid edge pixels in each image. The five-step algorithm described in the previous step is applied to calculate an optimal solution using those sampled edge pixels. The estimated solution is then tested on all valid edge pixels. The number of edge pixels whose distances to the silhouette projections of the geometrical model of the DP-IMN are less than a given threshold (e.g., 1 pixel) is recorded as L. This procedure is repeated a fixed number of times (e.g., 20 times) and the solution that yields the largest L is selected as the final estimation. shows an example of the initialization () and the final result ().
Automatic pose recovery of DLHs
The silhouette projections of the geometrical model of the DP-IMN into each image define a rectangular area. The projections of the DLHs in each image are located inside the associated rectangular area. To determine those edge pixels belonging to the projections of DLHs in ML images, the method reported in reference Citation[12] is modified for our purpose. A parallelepiped window, whose sizes are equal to the width of the rectangular area, is swept along the projected line of the estimated axis of the DP-IMN to find two locations which contain the maximum number of edge pixels and whose distance is greater than a pre-selected distance threshold T (e.g., the width of the rectangular area). However, unlike the method reported in reference Citation[12], no ellipse fitting is required, as our approach works directly on the extracted edge pixels.
The detected edge pixels of the DLHs in the ML image are helpful in locating a similar square window in the AP image as follows. In each detected location in the ML image, the centroid of the detected edge pixels inside the square window is calculated. Then, a point on the axis of the DP-IMN that is closest to the backward projection ray of the centroid is computed. Its projection into the AP image defines the location of a square window of the same size in the AP image.
Observing the wide variations in nail deformation, we opted for recovering the poses of the two DLHs one by one. In the following descriptions, we concentrate on pose recovery of the proximal hole. The same principle is applied to the distal hole, taking the orientation of the recovered axis of the proximal hole as the initial orientation of its axis.
Given the estimated axis of the DP-IMN, the initial transformation between the local COS of the geometrical model of the DLH and P-COS is obtained by taking the estimated axis of the DP-IMN in P-COS as the u axis and the normal of the ML imaging plane as the v axis. All points defined in the local COS of the geometrical model of the DLH can then be transformed to P-COS using this initial transformation. The task of recovery of the DLH is to resolve the rotation α and the translation δ of the DLH along the axis by fitting the geometrical model of the DLH to both images, which is solved iteratively using the IBMPP algorithm again as follows:
Let Hk be a set of NHk 2D edge pixels of the DLH projection detected from image Sk. Further, let Mt−1 be a set of NM model points
at iteration step t − 1. Now, in the iteration step t, we perform the following steps:
Simulating X-ray projection: In this step, we simulate the X-ray projection of the geometrical model of the DLH to remove invisible points. Let
be a set of NPk 2D projection points
obtained by simulating X-ray projection of the 3D model into image Sk. Normally, NPk ≪ NM. Thus, for each 2D projection point
, we know its associated 3D model point
.
Finding closest projection point: In this step, we try to find the closest neighbor edge pixel
of each 2D model projection point
.
Establishing 3D-2D correspondence: For each 2D matched pair
, calculate the backward projection ray
of the 2D edge pixel
. Then, for the ray
, calculate a 3D point pair
, where
is a point on the ray
that is closest to the 3D model point
of the model projection point
.
Estimating transformation: For all calculated 3D point pairs
, find an optimal local solution of all pose parameters by solving the following minimization problem:
where
is a constrained rigid transformation around the estimated axis of the DP-IMN determined by rotation angle α(t−1) and translation δ(t−1).
Updating pose: Update the pose of all model points
by
to
.
These steps are repeated until all pose parameters are converged.
The IBMPP algorithm can be regarded as a local minimum search algorithm. It is possible for our algorithm to stop at a local minimum of the cost function in equation (3). The global minimum may be well hidden among many poorer local minima, especially when the initialization is not so close to the global minimum. In our approach, this is handled by combining a genetic algorithm Citation[21] with the IBMPP algorithm. The genetic algorithm acts as a random generator for possible parameter sets that solve the minimization problem. shows an example of the initialization for pose recovery (top) and the final result after our approach is converged (bottom).
Figure 5. Example of pose recovery of a DLH by fitting its geometric model to the images. Top: The initial situation, where the thin white points are the detected DLH edge pixels and the thicker yellow points are the simulated X-ray projections of the DLH model. Bottom: The situation after the algorithm converges to the optimal solution. [Color version available online.]
![Figure 5. Example of pose recovery of a DLH by fitting its geometric model to the images. Top: The initial situation, where the thin white points are the detected DLH edge pixels and the thicker yellow points are the simulated X-ray projections of the DLH model. Bottom: The situation after the algorithm converges to the optimal solution. [Color version available online.]](/cms/asset/2cc43f90-a2f1-4928-8e5f-e4302da6ea5c/icsu_a_238697_f0005_b.gif)
Preliminary in vitro study
Study setup
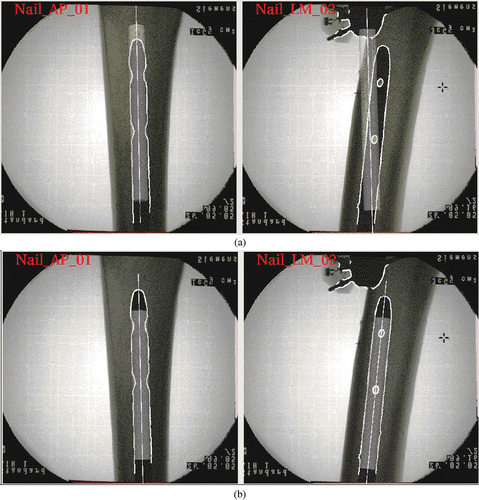
A preliminary in vitro study was conducted to analyze the accuracy and robustness of our approach. A SYNTHES® 9-mm solid titanium femoral nail (STRATEC Medical, Oberdorf, Switzerland) () was inserted with reaming into a cadaveric human femur and locked proximally. The inserted nail and the cadaveric specimen were treated as a single rigid body. An ISO-C C-arm (Siemens AG, Erlangen, Germany) was used to acquire fluoroscopic images for our experiments. Optoelectronically trackable marker shields containing infrared (IR) light-emitting diodes (LEDs) were attached to the C-arm, the cadaveric specimen, and the surgical tool to establish a local coordinate system for each entity. Real-time navigation was achieved through rigid-body coordinate transformations based on optoelectronic tracking (OPTOTRAK 3020, Northern Digital, Inc., Waterloo, Ontario, Canada) of the C-arm, the cadaveric specimen, and the surgical tools, as shown in . The ground truth of the DLH was obtained after image acquisition by inserting a custom-made steel rod through the hole and then digitizing both the top and bottom centers of the rod using a sharp pointer, as shown in . These two digitized points define the ground truth of the axis of the associated DLH.
Figure 6. The cadaveric bone and nail used in our preliminary in vitro experiments. [Color version available online.]
![Figure 6. The cadaveric bone and nail used in our preliminary in vitro experiments. [Color version available online.]](/cms/asset/d6cd6ea5-88be-43e7-aa4b-a70a99981884/icsu_a_238697_f0006_b.gif)
Figure 7. Experimental setup for image acquisition (left), and examples of acquired images (right). [Color version available online.]
![Figure 7. Experimental setup for image acquisition (left), and examples of acquired images (right). [Color version available online.]](/cms/asset/0e0371bf-2380-49c7-bf44-f79abda9fab5/icsu_a_238697_f0007_b.gif)
Figure 8. Experimental setup for obtaining ground truths by direct pointer-based digitization. The ground truth of the DLH was obtained after image acquisition by inserting a custom-made steel rod through the hole and then digitizing both the top and bottom centers of the rod using a sharp pointer. [Color version available online.]
![Figure 8. Experimental setup for obtaining ground truths by direct pointer-based digitization. The ground truth of the DLH was obtained after image acquisition by inserting a custom-made steel rod through the hole and then digitizing both the top and bottom centers of the rod using a sharp pointer. [Color version available online.]](/cms/asset/2b83c8cb-17aa-4a06-9cdc-7f8605478986/icsu_a_238697_f0008_b.gif)
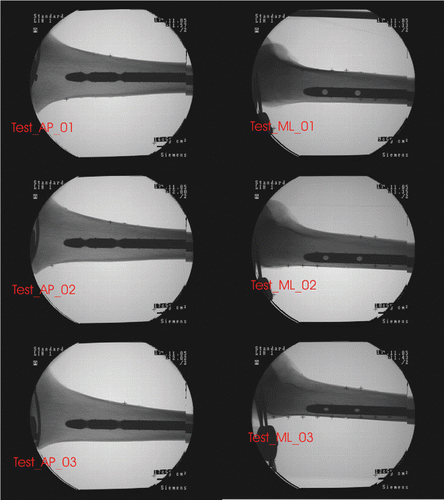
Six images, comprising a set of three generic AP images (Test_AP_01, Test_AP_02, and Test_AP_03) and a set of three generic ML images (Test_ML_01, Test_ML_02, and Test_ML_03) (see ), were acquired for our experiments. The first image in each set was taken as the reference position. The other two images in each set were acquired by further rotating the C-arm through increments of approximately 10° around the nail, each time starting from the reference position. All images were calibrated and registered to the local coordinate system of the cadaveric specimen using custom-made software Citation[15]. These six images were used to create nine pairs of images. In each case, a generic AP image was combined with a generic ML image to create a pair of images for the experiments described below.
Experiments
Convergence experiment
This experiment was designed to evaluate the converging rate of our approach. A trial is regarded as converged when the angular difference between the estimated axis of the DLH and the ground truths is less than 5°.
Accuracy and reliability experiments
The overall accuracy of our approach depends on the accuracies of both stages. This is why we did not evaluate the accuracies of these two stages separately. In the following experiments, possible factors that might affect the accuracy and reliability of our approach were further identified and evaluated:
Precision of DLH edge detection. After the sub-images that contained the projections of the DLHs were extracted, we could choose to extract the DLH edges by applying a classical Canny edge detector or by applying one based on an interpolation algorithm. Cubic splines Citation[22] were used to interpolate the sub-images to a higher resolution, then the Canny edge detector was applied so that we could extract the DLH edges with sub-pixel precision. shows examples of the detected DLH edges with (bottom row) and without (top row) interpolation.
Approximation accuracy of the geometrical model of the DLH. There should be a trade-off between the approximation accuracy of the geometrical model of the DLH and the gained accuracy of recovery. Two different geometrical models of the DLH, as shown in , were used in our experiments. The less accurate model used 18 points to approximate the inner circle and 12 points for the outer curve, while the other model used 36 points and 24 points for the inner circle and outer curve, respectively. We used more points to describe the inner circle because the information extracted from the ML image is more important in the recovery of DLHs than that extracted from the AP image.
Figure 10. The DLH edges extracted with different degrees of precision. Top: Edges extracted by applying a Canny edge detector without interpolation of the underlying sub-images. Bottom: Edges extracted by applying a Canny edge detector with interpolation of the underlying sub-images.
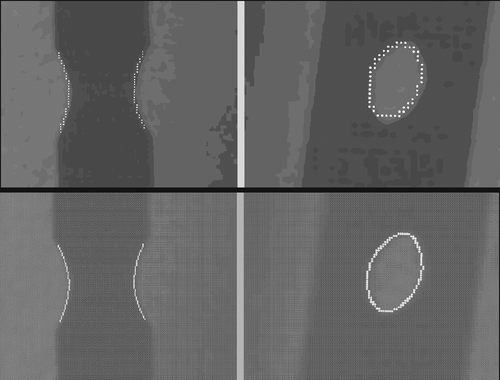
Figure 11. The geometrical model of the DLH with different approximation accuracies. Left: The less accurate model using 18 points to approximate the inner circle and 12 points for the outer curve. Right: The more accurate model using double the number of points in each case. [Color version available online.]
![Figure 11. The geometrical model of the DLH with different approximation accuracies. Left: The less accurate model using 18 points to approximate the inner circle and 12 points for the outer curve. Right: The more accurate model using double the number of points in each case. [Color version available online.]](/cms/asset/8bfaefa1-a341-4ec2-9af7-8d1d0250483b/icsu_a_238697_f0011_b.gif)
We designed and performed two experiments on the nine pairs of images described above to quantify the effect of these two factors on the overall accuracy and reliability of our approach. In each experiment, we applied our approach 10 times to each pair of images. Each time, we compared the estimated poses of the DLHs to the ground truths obtained by direct digitization. Two deviations were recorded: (a) angular deviation, which was defined as the angle between the estimated axis of the DLH and its associated ground truth; and (b) positional deviation, which was defined as the distance between two intersectional points, the first of which was defined by the intersection between the estimated axis of the DLH and the estimated axis of the DP-IMN, while the second was defined by the intersection between the digitized axis of the DLH and the estimated axis of the DP-IMN.
Results
All computation was done on a Sun Blade 100 workstation with 512 MB RAM and Creator-3D graphics board (Sun Microsystems, Schwerzenback, Switzerland). On average, for each input pair of images, it took approximately one minute to extract the “raw” edges from both the ML and AP images, another minute to detect the nail axis, and a further 1–2 minutes for recovery of the DLHs, depending on the approximation accuracy of the geometrical models of the DLHs. Note that the machine used was very old (due to some legacy source code), with computation power only equivalent to that of a low-end Pentium IV Intel PC. It is expected that the speed of the present approach will be much faster when implemented on a state-of-the-art machine.
Results of convergence experiment
The nail could be accurately localized in all trials using any pair of images, which was proved by visually checking the superimposition of the geometric model of the DP-IMN in both images. In all trials, the pose recovery stage converged after a number of iterations. The maximum number of iterations was seven and the minimum number was one.
Results of accuracy and reliability experiments
The results of the accuracy and reliability experiments are presented in Tables . With a fixed geometric model of the DLH, the improvement in angular accuracy was significant (P < 0.01) when the precision of the DLH edge detection was improved (comparing and ). This could be explained by comparing the DLH edges presented in the bottom row of with those in the top row of the same figure. The edges extracted with a higher precision approximate the DLH projections more accurately. In contrast, with a fixed precision of edge detection, the improvement in angular accuracy was less significant when the more accurate geometric model of the DLH was used (comparing and ). This might be explained by . The geometric model with a smaller number of points could already represent the DLH with a certain degree of accuracy, especially from the ML point of view (or the top view of the DLH). Comparing the positional deviation results reported in Tables , we found that the differences between different experiments are small. This might be explained by the high repeatability of automatic nail localization. When the DLH edges were detected with interpolation and the more accurate geometric model of the DLH was used (), a mean angular error of 0.5° (STD = 0.2°) and a mean translational error of 0.1 mm (STD = 0.0 mm) between the computed orientations and positions of the DLHs and the ground truths were observed.
Table I. Experiment results when DLH edges were detected without interpolation and the less accurate model of DLH was used.
Table II. Experiment results when DLH edges were detected with interpolation and the less accurate model of DLH was used.
Table III. Experiment results when DLH edges were detected with interpolation and the more accurate model of DLH was used.
Summary and Conclusions
We have presented an approach for fully automatic recovery of distal locking holes using two calibrated fluoroscopic images. The present approach does not depend on any special hardware setup. It can be integrated with any computer assisted surgery (CAS) system, including both freehand surgical navigation systems and medical robotics, providing two calibrated and registered fluoroscopic images of the DP-IMN are available (although we tested our algorithm using a freehand fluoroscopy-based navigation system). The requirements for these two fluoroscopic images are very flexible: They do not need to be perfect ML and AP images. The only condition that must be fulfilled is that the projections of DLHs are clearly identifiable from both images. Our approach is reliable, and works well even when there are projections from outliers such as interventional instruments and cables.
The present approach is very generic and can be applied to any type of nailing that satisfies the following conditions: (a) part of an IMN which contains two distal holes is a cylinder and its diameter is known or can be measured; (b) the geometries of the holes are known or can be measured; and (c) the axis of the hole intersects with the axis of the distal part of the IMN at one point. As the geometrical model of the nail is directly measured from the nail itself, the proposed approach does not depend on the Computer-Aided Design (CAD) model of the nail, which can only be obtained from the manufacturer. Modern standardized manufacturing technology makes it possible to apply the measurement from one product to all similar products from the same manufacturer, at least from the geometrical measurement point of view.
Regarding the geometrical model of the DLH, most previous work in the literature treated it as a cylinder of known radius and height Citation[8–12]. This simplified model prevented use of the information provided by the AP image. For example, in references Citation[8] and Citation[9], though both AP and ML images were used to detect the nail and to estimate the nail axis, only the ML image was used to recover the poses of the DLHs. We believe that the robustness and accuracy of pose recovery can be improved by accurately modeling the DLH, as in the present paper.
We have designed and conducted preliminary in vitro experiments to validate the present approach, and the results show that it is very reliable. Its accuracy is not too dependent on how the X-ray images are acquired, but does depend on how the edges of the DLHs are extracted and on the accuracy of the geometrical models of the DLHs. A mean angular error of 0.5° (STD = 0.2°) and a mean translational error of 0.1 mm (STD = 0.0 mm) demonstrate that it is possible to automatically recover the poses of the distal locking holes from two calibrated fluoroscopic images, and that the recovered poses are sufficiently accurate for intraoperative use.
References
- Brumback RJ, Uwagie-Ero S, Lakatos RP, Poka A, Bathon GH, Burgess A. Intramedullary nailing of femoral shaft fractures. Part II: Fracture-healing with static interlocking fixation. J Bone Joint Surg 1988; 70-A: 1453–1462
- Winquist RA, Hansen ST, Clawson DK. Closed intramedullary nailing of femoral fractures. A report of five hundred and twenty cases. J Bone Joint Surg 1984; 66-A: 529–539
- Wiss DA, Fleming CH, Matta JM, Clark D. Comminuted and rotationally unstable fractures of the femur treated with an interlocking nail. Clin Orthop 1986, 212: 35–47
- Krettek C, Mannß J, Miclau T, Schandelmaier P, Linnemann I, Tscherne H. Deformation of femoral nails with intramedullary insertion. J Orthop Res 1998; 16(5)572–575
- Skjeldal S, Backe S. Interlocking medullary nails – radiation doses in distal targeting. Arch Orthop Trauma Surg 1987; 106: 179–181
- Hofstetter R, Slomczykowski M, Krettek C, Köppen G, Sati M, Nolte L-P. Computer-assisted fluoroscopy-based reduction of femoral fractures and antetorsion correction. Comput Aided Surg 2000; 5: 311–325
- Suhm N, Jacob AL, Nolte L-P, Regazzoni P, Messmer P. Surgical navigation based on fluoroscopy – clinical application for computer-assisted distal locking of intramedullary implants. Comput Aided Surg 2000; 5: 391–400
- Zhu Y, Phillips R, Griffiths JG, Viant W, Mohsen A, Bielby M. Recovery of distal hole axis in intramedullary nail trajectory planning. Proc Inst Mech Eng [H] 2002; 216(5)323–332
- Malek S, Phillips R, Mohsen A, Viant W, Bielby M, Sherman K. Computer assisted orthopaedic surgical system for insertion of distal locking screws in intra-medullary nails: A valid and reliable navigation system. Int J Med Robotics Comput Assisted Surg 2005; 1(4)34–44
- Leloup T, Schuind F, Warzee N. Process for the acquisition of information intended for the insertion of a locking screw into an orifice of an endomedullary device. European Patent Application Number 04447153.0 2004
- Leloup T, El Kazzi W, Debeir O, Schuind F, Warzée N. Automatic fluoroscopic image calibration for traumatology intervention guidance. Proceedings of IEEE EUROCON 2005 – The International Conference on “Computer as a tool”. Belgrade, Serbia & Montenegro November 2005; Volume 1: 374–377
- Yaniv Z, Joskowicz L. Precise robot-assisted guide positioning for distal locking of intramedullary nails. IEEE Trans Med Imaging 2005; 24(5)624–635
- Langlotz F, Nolte L-P. Technical approaches to computer-assisted orthopedic surgery. Eur J Trauma 2004; 30(1)1–11
- Nolte L-P, Visarius H, Arm E, Langlotz F, Schwarzenback O, Zamorano L. Computer-aided fixation of spinal implants. J Image Guided Surg 1995; 1: 88–93
- Hofstetter R, Slomczykowski M, Sati M, Nolte L-P. Fluoroscopy as an imaging means for computer-assisted surgical navigation. Comput Aided Surg 1999; 4: 65–76
- Canny J. A computational approach to edge detection. IEEE Trans Pattern Anal 1986; 8(6)679–698
- Zheng G, Zhang X, Haschtmann D, Gédet P, Dong X, Nolte L-P. A robust and accurate two-stage approach for automatic recovery of distal locking holes in computer-assisted intramedullary nailing of femoral shaft fractures. Submitted for review 2006
- Guéziec A, Kazanzides P, Williamson B, Taylor RH. Anatomy-based registration of CT-scan and intraoperative X-ray images for guiding a surgical robot. IEEE Trans Med Imaging 1998; 17(5)715–728
- Fischler MA, Bolles RC. Random sample consensus: A paradigm for model fitting with applications to image analysis and automated cartography. Commun ACM 1981; 24(6)381–395
- Veldpaus FE, Woltring HJ, Dortmans LJ. A least-squares algorithm for the equiform transformation from spatial marker co-ordinates. J Biomech 1988; 21(1)45–54
- Goldberg DE. Genetic algorithms in search, optimization, and machine learning. Addison-Wesley, Reading, MA 1989
- Unser M, Aldroubi A, Eden M. Fast B-spline transforms for continuous image representation and interpolation. IEEE Trans Pattern Anal 1991; 13(3)277–285