Abstract
Objective: A comprehensive study was performed to evaluate the accuracy of a newly developed CT-free, intra-operative planning and navigation system for anterior spine surgery.
Materials and Methods: Instruments and an image intensifier were tracked using the SurgiGATE® navigation system. A laboratory study was performed on 27 plastic vertebrae. Fiducial markers were implanted in the vertebrae for accuracy evaluation purposes, and a dynamic reference base was placed on the vertebrae to establish a patient coordinate system (P-COS). Two fluoroscopic images were used for intra-operative planning. The graft bed plan was recorded in P-COS, followed by surgical formation of the graft bed, which was visualized. To evaluate the accuracy, the vertebrae were scanned with CT, and the markers were used to calculate an accurate paired-point registered transformation between the CT coordinate system and P-COS.
Results: Using the new SPO module, accurate planning and navigation of a resection of the vertebral body is possible using two fluoroscopic images. The overall mean error between the planned resection volume and the actual resection was 0.98 mm. In addition, the module can serve as an educational tool for training spine surgeons.
Conclusions: The new fluoroscopy-based system can be used safely for accurate performance of anterior resection during spondylodesis. New methods for safe and accurate registration during anterior spine surgery need to be developed.
Introduction
Treatment of patients with trauma and diseases of the spine Citation[1–3] remains an urgent problem in neurosurgery, trauma surgery, and orthopaedics. In recent years, surgical technologies have formed an increasingly large portion of the available methods for treating patients with spinal pathology. The most important elements of surgical treatment of such cases include reconstruction of the supporting structure and reliable stabilization of a spinal column in the proper position, permitting the effective restoration of lost functions at an early stage. So far, one of the main methods of reconstruction of such structure has been anterior spondylodesis with partial or complete substitution of an affected vertebral body using bone auto- or allografts Citation[4]. The limitations of conventional techniques lie in the difficulties of precise three-dimensional (3D) graft bed formation, in the high radiation exposure associated with spinal cord decompression, and in the uncomfortable combination of minimally invasive approaches and imaging intensifiers. As the minimally invasive endoscopic approach for treatment of spinal pathologies is routinely used by many spine surgeons and is characterized by a long learning curve, the need for sophisticated surgical education arises Citation[5].
Computer-assisted surgery has made tremendous progress during the last decade. Different systems are now available to perform image-guided surgery on various body parts Citation[6–8]. The so-called spine modules of these systems enable the precise introduction of image-guided drills, and allow computer-assisted placement of transpedicular screws for implantation of internal fixators Citation[5], Citation[8–10]. Most of the available CAS systems are dependent on pre-operative CT scans. Recently, surgical navigation based on single fluoroscopic images of the spine has become availabl Citation[9], Citation[11]. Whereas computer-assisted posterior instrumentation of spine fractures was successfully introduced as early as 1994 Citation[8], and was clinically proven to significantly reduce the morphological outliers of screw placements Citation[10], only a few papers concerning related approaches for anterior spinal navigation have appeared in the literatuCitation[5], Citation[12], Citation[13]. In particular, to our knowledge, there have been no published reports describing a navigated resection of a volume determined prior to surgery.
The goal of the work reported here was to evaluate a newly developed CT-free intra-operative planning and navigation system for minimally invasive spondylodesis of spine fractures.
Materials and methods
Navigation system
The study used the SurgiGATE® navigation system (PRAXIM MediVision, La Tronche, France) Citation[11] (). An optoelectronic camera (Optotrak 3020, Northern Digital, Inc., Waterloo, Ontario, Canada) mounted on a movable stand was used to track the position of the optical targets equipped with infrared light-emitting diodes (LEDs). These targets were attached to the surgical object, all surgical tools, and the image intensifier of a C-arm. Each set of LEDs defined a local coordinate system (COS). The rigid body transformation data provided by the camera were used to compute real-time intraoperative coordinate transformations between these systems. A Sun ULTRA 10 workstation (Sun Microsystems, Schwerzenbach, Switzerland) was chosen to handle all image processing and visualization tasks. It was connected to the standard video output of the C-arm using an off-the-shelf Osprey-150 video frame-grabber board (Osprey systems, Cary, NC). The workstation communicated with the tracking system through custom software and associated client/server architecture.
Image acquisition
Two fluoroscopic images taken from almost orthogonal directions – one from the anterior-posterior (AP) direction and the other from the lateral-medial (LM) direction – were acquired and automatically registered to the associated P-COS. A calibration procedure recently introduced by our group for fluoroscopic navigation Citation[14], which uses a 3D virtual reality technology based on a pinhole camera model, was established for each image. This allowed visualization of the virtual representation of the custom-made instrumentation as well as the planned object in 3D form, taking the acquired fluoroscopic image as the visualization background.
Intra-operative planning
Following image acquisition, four deep-seated landmarks (), which locate the four corners of the graft bed in the cranial-ventral, caudal-ventral, cranial-dorsal, and caudal-dorsal directions, respectively, were acquired with a bi-planar landmark reconstruction technique using multiple registered fluoroscopic images Citation[15]. A user-friendly manipulator is used to provide interactive planning of the size and shape of the graft bed by comparing the graft bed model projection with the underlying anatomical images. The graft bed was positioned inside the vertebral body to prevent the simulation of accuracy by performing a resection along anatomical borders, e.g., the endplates of the vertebral body (). In a second step, the shape and dimensions of the planned graft bed were used for precise preparation of the autograft from the donor area in an actual surgery.
Figure 2. Fluoroscopy image-based intra-operative planning of the graft bed. (a) Four deep-seated landmarks (displayed as two red spheres and two yellow spheres) which locate the four corners of the graft bed in the cranial-ventral, caudal-ventral, cranial-dorsal, and caudal-dorsal directions, respectively, are acquired with a bi-planar landmark reconstruction technique and used to guide the planning. (b) The surgeon can interactively adjust the position and orientation of the virtual graft bed model by comparing the projection of the graft bed model with the underlying anatomical images. [Color version available online.]
![Figure 2. Fluoroscopy image-based intra-operative planning of the graft bed. (a) Four deep-seated landmarks (displayed as two red spheres and two yellow spheres) which locate the four corners of the graft bed in the cranial-ventral, caudal-ventral, cranial-dorsal, and caudal-dorsal directions, respectively, are acquired with a bi-planar landmark reconstruction technique and used to guide the planning. (b) The surgeon can interactively adjust the position and orientation of the virtual graft bed model by comparing the projection of the graft bed model with the underlying anatomical images. [Color version available online.]](/cms/asset/d0ba0f4c-69ce-44fd-ac15-17277297e4c2/icsu_a_255148_f0002_b.gif)
Vertebrae
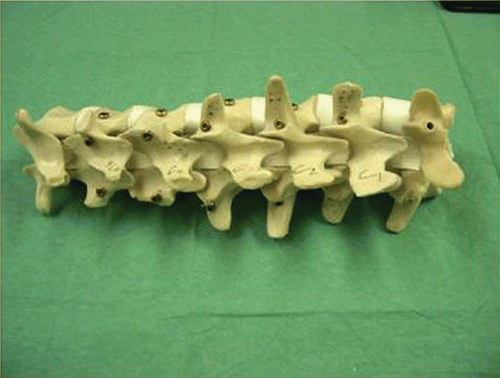
A total of 27 plastic vertebrae (Synbone AG, Malans, Switzerland) were used for our study. The same procedure was performed on each vertebra. The spine specimens were fixed semi-rigidly to a wooden working plate. For accuracy evaluation purposes, three titanium fiducial markers (Synthes, Oberkirch, Germany) were implanted into each vertebra (). The fiducials were fixed to both laminae and the spinal process of each vertebra to be operated on. In each case, a dynamic reference base (DRB) was rigidly placed in the pedicle of the vertebra to establish a patient coordinate system (P-COS). The coordinates of the aforementioned fiducial markers in the associated P-COS were recorded using an optoelectronically trackable pointer and were later used to set up a paired-point registration transform between the CT volume data coordinate system and the P-COS.
Surgical guidance
After planning, surgical actions such as the formation of the graft bed using a custom-made chisel and the placement of the stabilization devices were visualized for the surgeon by superimposing virtual instrument representations on the fluoroscopic images and . The distances between the instrument tip and each wall of the planned graft bed were calculated on the fly and presented to the surgeon so that he could formalize the graft bed exactly according to his plan. To validate the accuracy of this newly developed system, the formation of the graft bed was performed by two surgeons with different levels of experience in spine surgery.
Accuracy evaluation
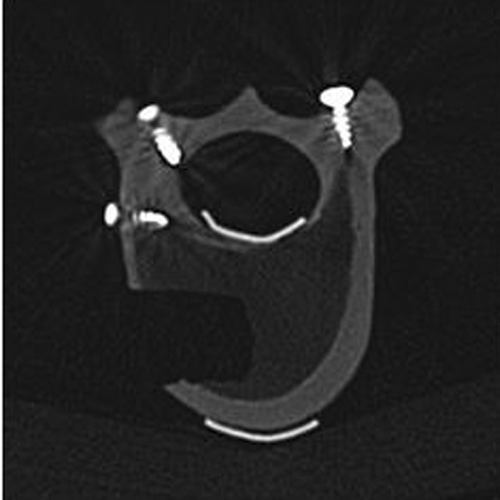
To evaluate the accuracy of the newly developed system, each operated vertebra was scanned by CT (Somatom Sensation-16, Siemens, Germany). The coordinates of the three aforementioned fiducial markers in the CT volume coordinate system were extracted and used together with their associated coordinates in the P-COS to calculate an accurate paired-point registration transformation between the CT coordinate system and P-COS. The boundary points of the formularized graft bed were extracted slice by slice from the CT volume data () and transformed to the P-COS using the calculated paired-point registration transformation. The distances between these points and the associated wall of the recorded graft bed plan were used to compute the accuracy of the newly developed system.
Statistical analysis
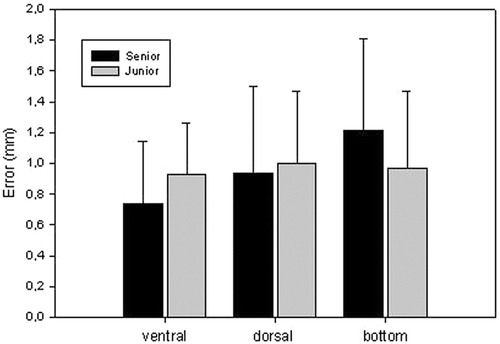
To evaluate whether the surgeon's experience influenced the degree of accuracy in image-guided resection, the accuracy results for the senior spine surgeon and the junior surgeon with no special education in spine surgery were analyzed statistically using Student's t-test.
Results
Using the newly developed SPO module, it was possible to perform intra-operative planning of the resection and the actual resection itself without any mistakes. In particular, the online information provided the surgeon with accurate guidance of all instruments during the surgery. Comparison of the planned and actual resections revealed a very high degree of precision. Anatomically, for each vertebra there are a total of five walls, i.e., the cranial, caudal, ventral, dorsal, and bottom walls, which are of relevance to the accuracy evaluation. We restricted our objective analysis to measuring errors in three of these walls, i.e., the ventral, dorsal, and bottom walls, as shown in . The errors in the cranial and caudal walls cannot be measured objectively because it is very difficult, if not impossible, to determine objectively the starting slice for the cranial plane and the ending slice for the caudal plane due to the partial volume effect. The results are presented in . The overall mean error was 0.83 mm for the ventral wall, 0.96 mm for the dorsal wall, and 1.13 mm for the bottom wall, while the overall mean error for all points was 0.98 ± 0.06 mm (mean ± standard deviation). During the surgical procedure, the newly developed system proved to be easy and safe to use.
Figure 7. The landmarks extracted from the formed graft bed and the planned graft bed for accuracy evaluation in one specimen (number A4). Colored spheres represent those landmarks extracted from CT volume data and transformed to the dynamic reference base (DRB) space using paired-point registration transformation. The rectangle represents the planned 3D graft bed in the DRB space as determined with our fluoroscopy-based planning and navigation system. [Color version available online.]
![Figure 7. The landmarks extracted from the formed graft bed and the planned graft bed for accuracy evaluation in one specimen (number A4). Colored spheres represent those landmarks extracted from CT volume data and transformed to the dynamic reference base (DRB) space using paired-point registration transformation. The rectangle represents the planned 3D graft bed in the DRB space as determined with our fluoroscopy-based planning and navigation system. [Color version available online.]](/cms/asset/22799d8a-9a8a-4265-a0da-976edb82688a/icsu_a_255148_f0007_b.gif)
Table I. Accuracy evaluation results. The LM columns show the number of points analyzed in each plane. The bottom row of the table shows the overall average error for the associated plane.
Interestingly, the different resection planes differed in their accuracy as compared to the original planning: the ventral and dorsal resection planes were more precise than the bottom plane. One possible reason for this may be the direction in which the surgeons had to work while preparing the bottom plane.
The new planning and navigation module fulfilled expectations as a training device for minimally invasive procedures. Accuracy analysis with respect to the surgical experience of the users showed that the level of surgical experience did not influence the accuracy of the resection, with no significant difference in accuracy being observed between the senior and junior spine surgeons ().
Discussion
This paper has described the evaluation of a newly developed planning and navigation module for computer-assisted graft bed formation during anterior spondylodesis. Major aspects of the investigation were general feasibility testing of the new method, evaluation of the reliability, safety and accuracy of the system, and determination as to whether the module could be used in surgical training. A laboratory study using plastic vertebrae and the original navigation systems and navigated instruments was conducted. After fluoroscopy-based planning and navigated resection, an independent accuracy evaluation was performed, with specific consideration of the surgeons' relative experience in spine surgery.
Over the last decade, computer-assisted surgery (CAS) has become increasingly important in orthopaedic surgery. Particularly in spine surgery, a high standard of accuracy is demanded due to the risk of iatrogenic damage to neural or vascular structures. Several studies have demonstrated that CAS improves accuracy in posterior instrumentation Citation[16–19]. Particularly demanding surgical procedures such as C1/C2 transarticular screw fixatio Citation[20], Citation[21] and transpedicular screw insertion into the cervical Citation[20], Citation[21] and thoracic spi Citation[16], Citation[22] showed improved accuracy if CAS techniques were used.
The main reason for using the CAS technique in endoscopic spine surgery is to provide online information regarding the current position of surgical instruments in relation to vulnerable structures like the spinal cord. In the present study, the newly designed planning and navigation module provided the required high accuracy during the surgical procedure. The overall error for all analyzed points was determined as a difference of 0.98 mm between the planned resection and that which was actually performed. This discrepancy is almost similar to the errors (<1.6 mm) reported earlier by Assaker et al. for frame-based surface matching registration in anterior spine surgery Citation[5], Citation[12]. Klein et al. showed that their error when using a navigated Kerrison punch for foraminotomy ranged between 1.4 and 1.8 mm Citation[13]. In conclusion, it can be stated that the new module is accurate and safe with a very low and comparable degree of error.
With minimally invasive endoscopic approaches to the thoracic and thoracolumbar spine becoming more common in the treatment of several degenerative and traumatic disorders of the spine, many surgeons who were familiar with the “old-fashioned” open approach techniqu Citation[5], Citation[12] are having to relearn the procedures. As the new endoscopic technique is based on a two-dimensional display of the operation site, which involves a long learning curve and is accompanied by the need for intensive training, it seemed reasonable to adapt routinely used CAS techniques for posterior instrumentation of the spine to anteriorly performed procedures such as intervertebral body fusion or spondylodesis following fractures of the thoracolumbar spine Citation[5], Citation[12].
While the use of CT-based navigation is possible in posterior instrumentatioCitation[16], Citation[17], Citation[20] because the posterior parts of the vertebra are exposed during the surgical procedure and can thus be used for surface matching to accurately register the vertebra for the navigation system, the situation in minimally invasive endoscopic approaches is completely different. Exposure of the vertebral body is limited to the minimum extent necessary to perform surgery. Therefore, the use of surface-matching-dependent CT-based navigation in endoscopic spine surgery is not possible. An alternative way to acquire data for the navigation system is to use a fluoroscopy-based system. These systems are dependent on the acquisition of two perpendicular X-ray images and display the spine in a 2D manner (in contrast to CT-based navigation, which is capable of displaying the spine in a 3D manner). Registration must also be performed in CT-based navigation procedures, but the fixation of a reference device to the spine during anterior procedures is much more difficult and susceptible to influences which may disturb navigation. Assaker et al. Citation[5], Citation[12] tried to solve this problem by attaching a specially designed titanium frame to the vertebra that was to be operated on. This enabled surface matching to be performed, using the rigidly attached frame as effectively part of the vertebra. Although this procedure is possible, it requires a second surgical approach to the dorsal section of the vertebra or percutaneous fixation of a guide wire. In addition, the requirements for this procedure are very complex as the patient has to be transferred to the CT and back to the operating theater. For this reason, ongoing development is focused on alternative referencing techniques, e.g., intra-thoracic tracking using the endoscope or 2D-3D matching. Of these, 2D-3D registration in particular may result in a significant improvement in the presented software module, as it will allow 3D display of the operation site and the surgical instruments.
In summary, we were able to demonstrate as a first step that the new planning and navigation module works in an accurate, safe and reproducible way during anterior resection, and we hypothesize that the minimally invasive surgical approach and protocol using navigated instruments during anterior surgical procedures is feasible, will improve security for navigated debridement of injured bony/ligamentous structure, and will both provide and enhance accuracy of graft bed formation and implant position. In addition, we hypothesize that the module can be used as an education device during the training of spine surgeons.
If the ongoing development is able to solve the problems of patient registration in anterior spine surgery, this method should become established as an accurate and safe procedure in spine surgery, offering reduced exposure to radiation for the patient and operating room s Citation[23], Citation[24]. Now that the feasibility of the module itself has been proven in the presented study, we plan to evaluate the SPO module in a more clinical context using fresh human cadavers in the near future.
Acknowledgment
This research was supported by AO Research Grant 03-R72.
References
- Kinzl L, Arand M, Mesmmert M, Mutschler W. [Ventral stabilization of thoracic and lumbar spinal injuries] [German]. Langenbecks Arch Chir Suppl Kongressbd 1992; 293–296
- Buhren V. [Injuries of the thoracic and lumbar spine] [German]. Chirurg 2001; 72: 865–878
- Chapman JR, Anderson PA. Thoracolumbar spine fractures with neurologic deficit. Orthop Clin North Am 1994; 25: 595–612
- Muschler GF, Negami S, Hyodo A, Gaisser D, Easley K, Kambic H. Evaluation of collagen ceramic composite graft materials in a spinal fusion model. Clin Orthop Relat Res 1996; (328): 250–260
- Assaker R, Cinquin P, Cotten A, Lejeune JP. Image-guided endoscopic spine surgery: Part I. A feasibility study. Spine 2001; 26: 1705–1710
- DiGioia AM, III, Jaramaz B, Plakseychuk AY, Moody JE Jr, Nikou C, LaBarca RS, Levison TJ, Picard F. Comparison of a mechanical acetabular alignment guide with computer placement of the socket. J Arthroplasty 2002; 17: 359–364
- Joskowicz L, Milgrom C, Simkin A, Tockus L, Yaniv Z. FRACAS: A system for computer aided image-guided long bone fracture surgery. Comput Aided Surg 1998; 3: 271–288
- Nolte LP, Zamorano LJ, Langlotz F, Jiang Z, Wang Q, Berlemann U. A novel approach to image guided spine surgery. Proceedings of 4th International Conference on Visualization in Biomedical Computing (VBC '96), RA Robb. Hamburg, Germany September, 1996; 564–573, SPIE 1994;2359:
- Berlemann U, Langlotz F, Langlotz U, Nolte LP. [Computer-assisted orthopedic surgery. From pedicle screw insertion to further applications] [German]. Orthopade 1997; 26: 463–469
- Laine T, Lund T, Ylikoski M, Lohikoski J, Schlenzka D. Accuracy of pedicle screw insertion with and without computer assistance: A randomised controlled clinical study in 100 consecutive patients. Eur Spine J 2000; 9: 235–240
- Nolte LP, Slomczykowski MA, Berlemann U, Strauss MJ, Hofstetter R, Schlenzka D, Laine T, Lund T. A new approach to computer-aided spine surgery: Fluoroscopy-based surgical navigation. Eur Spine J 2000; 9(Suppl 1)S78–S88
- Assaker R, Reyns N, Pertruzon B, Lejeune JP. Image-guided endoscopic spine surgery: Part II: Clinical applications. Spine 2001; 26: 1711–1718
- Klein GR, Ludwig SC, Vaccaro AR, Rushton SA, Lazar RD, Albert TJ. The efficacy of using an image-guided Kerrison punch in performing an anterior cervical foraminotomy. An anatomic analysis. Spine 1999; 24: 1358–1362
- Hofstetter R, Slomczykowski M, Sati M, Nolte LP. Fluoroscopy as an imaging means for computer-assisted surgical navigation. Comput Aided Surg 1999; 4: 65–76
- Grützner PA, Zheng G, Langlotz U, von Recum J, Nolte LP, Wentzensen A, Widmer KH, Wendl K. C-arm based navigation in total hip arthroplasty - background and clinical experience. Injury 2004; 35(Suppl 1)S-5
- Arand M, Hartwig E, Hebold D, Kinzl L, Gebhard F. [Precision analysis of navigation-assisted implanted thoracic and lumbar pedicle screws. A prospective clinical study] [German]. Unfallchirurg 2001; 104: 1076–1081
- Gebhard F, Weidner A, Liener UC, Stockle U, Arand M. Navigation at the spine. Injury 2004; 35(Suppl 1)S-45
- Reichle E, Morlock M, Sellenschloh K, Eggers C. [Definition of pedicle malposition. Primary stability and loosening characteristics of pedicle screws in relation to position: spongious anchoring, cortical anchoring, perforation and malposition] [German]. Orthopade 2002; 31: 402–405
- Reichle E, Sellenschloh K, Morlock M, Eggers C. [Placement of pedicle screws using different navigation systems. A laboratory trial with 12 spinal preparations] [German]. Orthopade 2002; 31: 368–371
- Goffin J, Van Brussel K, Martens K, Vander SJ, Van Audekercke R, Smet MH. Three-dimensional computed tomography-based, personalized drill guide for posterior cervical stabilization at C1-C2. Spine 2001; 26: 1343–1347
- Richter M, Cakir B, Schmidt R. Cervical pedicle screws: Conventional versus computer-assisted placement of cannulated screws. Spine 2005; 30: 2280–2287
- Amiot LP, Lang K, Putzier M, Zippel H, Labelle H. Comparative results between conventional and computer-assisted pedicle screw installation in the thoracic, lumbar, and sacral spine. Spine 2000; 25: 606–614
- Battaglia TC, Tannoury T, Crowl AC, Chan DP, Anderson DG. A cadaveric study comparing standard fluoroscopy with fluoroscopy-based computer navigation for screw fixation of the odontoid. J Surg Orthop Adv 2005; 14: 175–180
- Linhardt O, Perlick L, Luring C, Stern U, Plitz W, Grifka J. [Extracorporeal single dose and radiographic dosage in image-controlled and fluoroscopic navigated pedicle screw implantation] [German]. Z Orthop Ihre Grenzgeb 2005; 143: 175–179
![Figure 1. Surgical navigation system setup. Left: The SurgiGATE® navigation system. Top right: The optoelectronically trackable C-arm. Bottom right: Surgical tools including the dynamic reference base (DRB), chisel, gravity device, and pointers. [Color version available online.]](/cms/asset/3d914a03-7d12-4f19-96f5-cfe99e9fb840/icsu_a_255148_f0001_b.gif)
![Figure 4. Resection of the volume of the vertebral body as previously planned. The instrument is inside the resection volume. [Color version available online.]](/cms/asset/334e6d63-0235-40db-9ebd-dd1dc579781f/icsu_a_255148_f0004_b.gif)
![Figure 5. Resection of the volume of the vertebral body as previously planned. The instrument is outside the resection volume. [Color version available online.]](/cms/asset/1b5f2036-b43e-495b-bb18-a1bb15e84aa9/icsu_a_255148_f0005_b.gif)