Abstract
Objective: Computer-assisted graft implantation may contribute to achieving biological joint replacement in the future. The purpose of this experimental study was to evaluate the feasibility and accuracy of a series of computer-assisted graft implantations into human cadaver ankle joints.
Methods: Three-dimensional graft models of virtually planned corresponding tibial and talar defects were created from bovine cancellous bone. A platform for computer-assisted surgery (CAS) was set up to implant the grafts. Registration was performed by pair-point matching with anatomical landmarks. In the case of insufficient registration accuracy, artificial landmarks were used for registration. Eight grafts (four tibial, four talar) were implanted in four human cadaver ankle joints. Postoperative CT was used for outcome analysis. The following criteria of accuracy were defined: macroscopic quality of implant fit; quality of the sagittal and coronar joint surface; and quality of the undersurface of the graft in relation to the base of the defect.
Results: No technical complications were observed during computer-assisted graft implantation. Clinically acceptable accuracy was achieved in 6 of 8 graft implantations, with implant failure occurring at the tibial and talar location in one ankle joint. In total, 25 of 32 criteria of accuracy were achieved: 6/8 for macroscopic implant fit; 5/8 for quality of the sagittal joint surface; 7/8 for quality of the coronar joint surface; and 7/8 for quality of the undersurface of the graft. Registration with anatomical landmarks did not achieve sufficient accuracy in 4 of 8 cases, whereas registration with artificial landmarks was successful in all these cases.
Conclusions: We demonstrated the feasibility and accuracy of computer-assisted graft implantation for tissue-engineered replacement of the human ankle joint. However, we cannot recommend the present type of registration by pair-point matching with anatomical landmarks due to the considerable inaccuracies. The focus should be on the improvement of non-invasive registration techniques and methods for evaluating postoperative outcome.
Introduction
Biological joint replacement is a long-standing vision of orthopaedic and trauma surgery. Primary implantations of artificial joint prostheses into hip Citation[1] and knee Citation[2] joints show good and reliable long-term results. However, complications are more frequently observed in other joints, particularly the ankle Citation[3]. In post-traumatic osteoarthritis (OA) of the ankle joint, the clinical results of total endoprosthesis are rather unsatisfying and high rates of loosening have been reported Citation[4]. Arthrodesis of the upper ankle joint usually alleviates the pain but also favors the development of secondary problems, e.g., OA of adjacent joints, functional loss, and persistent change of the gait pattern Citation[5]. The incidence of post-traumatic OA of the ankle joint (79.5%) is far higher compared to that of the hip (1.6%) and knee (9.8%) joints Citation[6], and the condition also affects a higher proportion of young patients.
Surgical treatment aims at preservation of ankle joint function. To date, only the early stages of OA can be treated by reconstructive techniques. Microfracturing Citation[7], retrograde drilling Citation[8], cancellous bone grafting Citation[9], and transplantation of osteochondral plugs Citation[10] or autologous chondrocytes Citation[11] are effective treatment options for circumscribed high-grade chondral and osteochondral defects. Corresponding tibial and talar lesions (“kissing lesions”) represent a particular therapeutic challenge. Subchondral “kissing lesions” are observed in approximately 9% of patients following severe ankle trauma Citation[12].
Post-traumatic OA of the ankle joint highlights the need for alternate surgical treatment options. Ideally, a functional replacement would consist of biological material, but autologous transplantation techniques are restricted with respect to the size of the implant. Tissue engineering may contribute significantly to the conception of biological joint prostheses in the future. Currently, efforts are being made to generate large tissue-engineered osteochondral autografts for application in weight-bearing locations Citation[13], Citation[14]. The feasibility of computer-assisted design and manufacture of grafts, defect debridement, graft implantation and outcome evaluation was successfully demonstrated for the human ankle joint in a pilot study Citation[15].
Nowadays, CAS techniques are broadly used in the field of orthopaedic and trauma surgery. In spine surgery, CAS improves the accuracy of pedicle screw positioning Citation[16], and other standard CAS applications are pelvic surgery Citation[17] and joint arthroplasty Citation[18]. To date, application of CAS at the ankle joint is not so common. Rosenberger et al. Citation[9] performed computer-assisted retrograde drilling of osteochondritic talar lesions, but there have been no reports of computer-assisted implantation of an artificial ankle joint prosthesis. However, we previously demonstrated in a pilot study the feasibility of computer-assisted ankle joint replacement using bio-engineered autografts in a plastic ankle joint model and a human cadaver ankle joint Citation[19].
The combination of tissue engineering and CAS reveals novel surgical treatment options for post-traumatic OA of the ankle joint. For the purpose of this study, we created an experimental set-up that can be applied in clinical practice. The logistics of the surgical procedure were an important aspect of the study, and we intended to perform computer-assisted defect debridement and graft implantation in a single-stage procedure. The aim of our study was to evaluate the feasibility and accuracy of computer-assisted graft implantation for tissue-engineered replacement of the human ankle joint.
Methods
Graft design
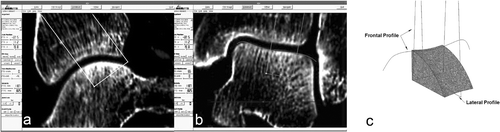
In consideration of the hinge-like articulation of the ankle joint, a rotational symmetric joint was assumed in the region of interest for joint replacement. A 3D model of the ankle joint was created on the basis of preoperative CT data as described previously Citation[19]. Interactive planning of joint replacement consisted of four steps: determination of the joint axis, determination of the sagittal graft profile (), determination of the coronar graft profile (), and 3D visualization of the graft design (). The 3D visualization was performed with the CAD software SolidWorks® (SolidWorks Corporation, Concord, MA). The CAD data of the planned graft design were transferred to a computer-assisted manufacturing (CAM) device (Picomax® 51, Fehlmann AG, Seon, Switzerland), and the grafts were custom-milled from bovine cancellous bone (Tutogen Inc., Neunkirchen, Germany) (). The accuracy of this technique was proven previously by members of the study group Citation[15]. We did not use processed tissue-engineered osteochondral grafts, since surgery was performed on human cadaver ankle joints. The grafts were manufactured 1.0 mm larger in each plane to favor press-fit implantation.
CAS platform
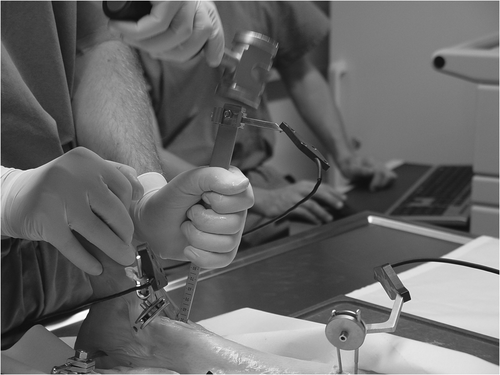
The CAS platform “MARVIN” was set up as described previously Citation[15]. The platform was created at the MEM Research Center for Orthopaedic Surgery (University of Bern) and is not commercially available. It consists of a C++ based framework library (base library, platform library, module library and external services). The platform application depends on the module used. Modules for segmentation and pair-point matching were established on the platform. The platform was used for preoperative planning and surgical navigation. MARVIN was run on a standard PC (Linux 2.6) with an Optotrak® 3020 high-resolution optical tracking system (Northern Digital Inc., Waterloo, Ontario, Canada). LED markers were attached to the tibia and talus via Schanz screws, and also to every surgical tool used. We designed a special set of three chisels for computer-assisted ankle arthroplasty: one chisel with a blade angulation of 90°, one with a straight blade and a bent 10-mm tip (45°), and one with a straight blade and a straight 9.5-mm tip.
Registration
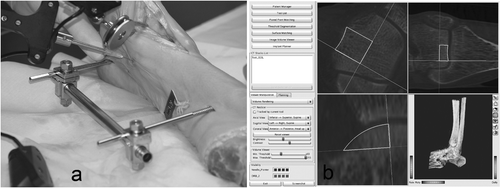
Registration was based on preoperative CT scans of the ankle joints. The slice thickness was 0.6 mm (200 mAs, 120 kV). A pointer was used for registration. Bony talar landmarks for pair-point matching were the medial and lateral shoulder of the talus, the most anterior margin of the talus (talo-navicular joint), and the anterior-medial border of the talus. Bony tibial landmarks were the anterior-medial and anterior-lateral border of the joint surface. Pair-point matching with anatomical landmarks was performed (). The accuracy of registration was set at <1.0 mm, which is an accepted threshold in clinical practice for joint surgery. Three small titanium screws (artificial landmarks) were implanted into both the distal tibia and the talus prior to the CT scan. In the case of insufficient accuracy of registration after pair-point matching with anatomical landmarks, we performed pair-point matching with artificial landmarks. Registration with artificial landmarks was used as a replacement technique for failed registration with anatomical landmarks; it was not the purpose of this study to compare the two techniques.
Defect debridement and graft implantation
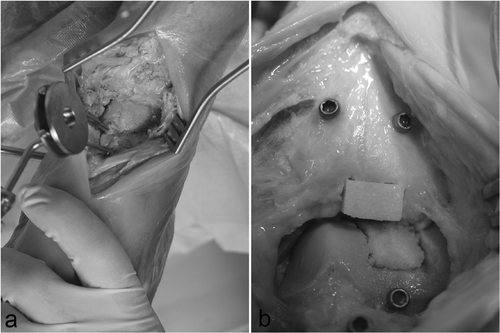
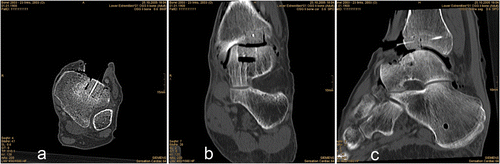
A 5.0-mm Schanz screw was implanted medially at both the distal tibia and talus. The ankle joint was distended with a longitudinal bar to improve visualization (external fixation), and LED markers were attached to separate Schanz screws that were implanted in the tibia and talus. An anterior approach to the ankle joint was performed. CAS tools were calibrated and calibration was checked prior to each procedure. Registration was performed until the required accuracy was achieved. Preoperative planning of the defects was performed on the CAS platform (MARVIN), and the contours of the defect were visualized on the CAS monitor. All surgical tools were continuously tracked with the camera and their current positions were visualized in the CT volume on the monitor. Eight defects (four tibial, four talar) were created in four human cadaver ankle joints (). The defects were created with the CAS chisels according to the preoperative planning. Surgery could be tracked online on the CAS monitor. The bone grafts were press-fit implanted with cautious use of a pestle. shows the operative site after completion of computer-assisted joint replacement. The Schanz screws were then removed and the wound was closed.
Outcome evaluation
Accuracy of graft implantation was analyzed on postoperative CT scans using an approved 19-inch monitor and special PACS software (JVision 3.3.16, Tiani Medgraph AG, Vienna, Austria). Slice thickness was 2.0 mm (200 mAs, 120 kV). Steps and gaps (range) at the implantation site were measured manually on all slices of the sagittal, coronar and axial reconstruction planes (). Two independent examiners performed the manual measurements. Inter-observer reliability was determined and mean values of the two measurements were used for outcome analysis. A set of criteria (1–4) was defined in order to specify the quality of the graft implantation. Definition of criteria was based on experimental and clinical studies on the etiology of post-traumatic OA of the ankle joint Citation[20–23]. Particular regard was given to the current state of scientific knowledge concerning the importance of joint congruency. The maximal values of steps and gaps in each plane were considered for evaluation of these criteria:
Macroscopic quality of implant fit: acceptable if the graft could be press-fit implanted and was properly held in place;
Quality of the sagittal joint surface: acceptable if no step larger than 1.0 mm and no gap larger than 2.0 mm was detected;
Quality of the coronar joint surface: acceptable if no step larger than 2.0 mm and no gap larger than 3.0 mm was detected;
Quality of the undersurface of the graft in relation to the base of the defect: acceptable if no gap larger than 4.0 mm was detected.
Results
Computer-assisted planning and manufacture of the grafts was successful in all cases without technical complications. The feasibility and accuracy of this procedure was recently demonstrated in trial operations on plastic ankle models and a human cadaver ankle joint Citation[15], Citation[19]. Data from preoperative planning were successfully transferred to the CAS platform. Also, the CAS platform and the CAS tools were fully functional throughout all surgical procedures.
Pair-point matching with anatomical landmarks failed to achieve the required accuracy of registration in 4 of 8 cases (2 ankles). In these cases, the required accuracy of registration was achieved by pair-point matching with artificial landmarks (titanium screws). A total of 25 of 32 (78.1%) criteria of accuracy were achieved: 6/8 for macroscopic implant fit; 5/8 for quality of the sagittal joint surface; 7/8 for quality of the coronar joint surface; and 7/8 for quality of the undersurface of the graft in relation to the base of the defect.
shows the results of the four graft implantations after registration by pair-point matching with anatomical landmarks (2 ankles). Macroscopic implant fit was achieved at one tibial and one talar location. Macroscopic implant failure occurred at the other two locations because the defects were too large. Consequently, the grafts could not be press-fit implanted and had to be held in place by a Kirschner wire. The quality of the sagittal joint surface was acceptable in 2 of 4 locations: The two tibial locations did not achieve the required quality of the sagittal joint surface. The quality of the coronar joint surface was achieved in 3 of 4 locations: One tibial location did not achieve the required surface quality of the coronar joint surface. The quality of the undersurface of the graft in relation to the base of the defect was acceptable in 3 of 4 locations: One talar location did not achieve the required quality of the undersurface of the graft in relation to the base of the defect. A total of 10 of 16 (62.5%) criteria of accuracy were achieved after registration by pair-point matching with anatomical landmarks. One of 4 grafts (Talus 2) achieved 4 of 4 criteria of accuracy corresponding to a clinically acceptable result. Inaccuracies were significantly (p < 0.05) higher at the posterior implantation sites (e.g., Talus 1).
Table I. Accuracy of graft implantation after registration by pair-point matching with anatomical landmarks.
shows the results of the four graft implantations after registration by pair-point matching with artificial landmarks (2 ankles). Macroscopic implant fit was achieved at all locations, and the quality of the sagittal joint surface was acceptable in 3 of 4 locations: One tibial location did not achieve the required quality of the sagittal joint surface. The quality of the coronar joint surface and of the undersurface of the graft in relation to the base of the defect was acceptable in all locations. A total of 15 of 16 (93.8%) criteria of accuracy were achieved after registration by pair-point matching with artificial landmarks. Three of 4 grafts achieved 4 of 4 criteria of accuracy corresponding to a clinically acceptable result. Inaccuracies were significantly (p < 0.05) higher at the posterior implantation sites (e.g., Tibia 4), and in one case cortical infraction of the anterior border of the tibial defect was observed (Tibia 4).
Table II. Accuracy of graft implantation after registration by pair-point matching with artificial landmarks.
Accuracy of graft implantation was significantly (p < 0.01) higher after registration by pair-point matching with artificial landmarks than after registration by pair-point matching with anatomical landmarks. Inter-observer reliability of the postoperative CT measurements was 0.95 (kappa index).
Discussion
This study demonstrates the feasibility and accuracy of computer-assisted bone graft implantation for tissue-engineered replacement of the human ankle joint. It is also the first study to evaluate a series of computer-assisted graft implantations into human ankle joints.
Accuracy of registration by pair-point matching with artificial landmarks was superior to accuracy of registration by pair-point matching with anatomical landmarks. We postulate that a main reason for this was imprecise correspondence of bony landmarks as defined on the preoperative CT (image world) to the anatomical landmarks in situ (real world). Indeed, symmetry of the talar surface may favor inaccuracies in pair-point matching with anatomical landmarks due to a lack of characteristic bony structures to match. Accuracy of registration is particularly relevant in surgical fields of limited size. The ankle joint is a rather small joint compared to the hip and knee, and small deviations, e.g., at the talar dome, seem to lead to considerable inaccuracies in registration by pair-point matching with anatomical landmarks. Another reason for inaccuracy of registration by pair-point matching with anatomical landmarks was undoubtedly the limitation of the surgical approach. Characteristic anatomical landmarks (e.g., lateral process of the talus, tip of lateral and medial malleolus) were not accessible, since the surgical approach was kept as minimally invasive as possible. Use of a needle pointer did not improve accuracy of registration, since visual control of exact correspondence is not available with percutaneous use of the needle pointer. We did not observe failure of registration by pair-point matching with artificial landmarks; however, this represents an invasive registration technique that potentially–in cases where arthroscopy is not performed–requires additional surgery (the implantation of rigid bodies, e.g., screws, prior to the CT scan).
There were no technical complications in CAS, though visualization of the surgical tools sometimes failed initially. To solve this problem, we designed a clamp for the LED markers that could be adjusted in multiple directions. Inaccuracy of graft implantation was mainly localized to the posterior implantation sites and was related to specific problems with the surgical technique. The anterior approach was limited, since a minimally invasive procedure is desired in clinical practice, but this approach did not permit working perpendicular to the joint surface at far posterior locations. As a result, some tilting or sliding of the CAS tools (particularly of the chisels) may have occurred. We conclude that a tibial or fibular osteotomy may be necessary for the treatment of far posterior lesions in order to guarantee accurate control of the CAS tools Citation[10]. In the case of very hard bone we recommend drilling the corners of the defect. The use of a milling device for debridement of the defect appears to be particularly satisfactory: A round contour avoids fracture lines originating from the corners, and possibly debridement of the defect is more accurate. In our series, graft implantation after registration by pair-point matching with artificial landmarks was more accurate than after similar registration with anatomical landmarks. However, both registration techniques achieved the required accuracy (<1.0 mm). The difference in accuracy was probably related to the small number of cases, since we were aware of no technical explanation. Sugano et al. Citation[24] achieved accuracy of surface-based registration of 1.2 mm for the pelvis and 1.4 mm for the femur, and accuracy of 0.96 mm was described for surface-based registration in CT-based spine surgery Citation[25]. We achieved comparable accuracies for pair-point matching with anatomical landmarks at the ankle joint. However, in only half of our cases was the accuracy of registration below 1.0 mm. The improvement in non-invasive registration methods represents a major challenge Citation[26], Citation[27]. Accurate registration is a premise for anatomical reconstruction of the joint surface. Integrity of the joint surface seems to be of particular relevance for the ankle joint Citation[20], and surface matching possibly improves accuracy of registration. However, preoperative CT is usually required and surface models must be established by segmentation. In contrast to the knee joint, the technique is not available for routine application at the ankle joint. C-arm-based registration methods are the subject of current developments Citation[28], and intraoperative use of the C-arm offers some advantages: real-time CT data are available for segmentation and creation of a 3D surface model; registration is non-invasive; and the accuracy of graft implantation can be controlled intraoperatively by multiplanar reconstructions.
The application of two types of pair-point matched registration was a limitation of our study. Pair-point matching with anatomical landmarks was unsuccessful in 4 of 8 cases, and pair-point matching with artificial landmarks was substituted. It was not the purpose of the study to compare two types of pair-point matched registration; however, we believe the study was not significantly affected by this limitation, since the registration was successful in all cases and only one registration technique (pair-point matching) was used. We defined corresponding tibial and talar defects, though the location of the defects was not strictly standardized. Differences in defect location and also intra-individual anatomic differences might have influenced our results, but we do not assume an essential influence on our results by these limitations. The procedure was planned and performed by an experienced orthopaedic and trauma surgeon (W. K.). Our results are not comparable to those of previous studies, since no comparable data exist on computer-assisted joint replacement procedures for the human ankle.
In clinical practice we recommend performing diagnostic arthroscopy of the ankle joint prior to joint replacement by tissue-engineered bone grafts. The indication can be confirmed and the required cell source (e.g., chondrocytes, periosteal cells) harvested for tissue engineering of autologous osteochondral grafts. Mesenchymal cells may be extracted via bone marrow puncture, and rigid bodies may be implanted via stab incisions during this procedure. Since MRI does not reliably distinguish between higher grades of chondral lesions Citation[29], diagnostic arthroscopy improves and facilitates the design of the joint replacement. Information about the localization of chondral lesions can be imported into the CAS platform, e.g., with the use of a pointer. These data can be correlated with the preoperative MRI, which reliably detects subchondral lesions Citation[30]. We see some major problems that need to be overcome prior to clinical iteration of the proposed technique: 1) improvement of diagnostic tools for planning of the procedure; 2) improvement of non-invasive registration techniques; and 3) improvement of the surgical technique.
Furthermore, tissue-engineered osteochondral autografts are an important component of biological joint replacement. Schaefer at al. Citation[13] demonstrated osteochondral remodeling of bioabsorbable scaffolds up to 175 cm3 in size after press-fit implantation in weight-bearing locations of the rabbit knee. Absorption of bone marrow-derived cells promoted structural remodeling. Growth factors have the potential to improve vascularization, integration and remodeling of tissue-engineered osteochondral grafts Citation[31]. Current techniques for tissue engineering do not yet meet the demands of joint replacement in clinical application Citation[32]. However, we demonstrated the clinical feasibility and accuracy of graft implantations in the human ankle joint.
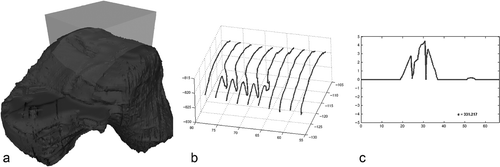
Post-traumatic arthritis may emerge as a result of pathological mechanical impact to the cartilage caused by direct trauma, incongruity Citation[33] and instability of the joint Citation[34]. Our criteria for accuracy of graft implantation were derived from experimental data Citation[22], Citation[33], Citation[35]. Elasticity of hyaline cartilage in the knee joint may compensate for a step of up to 1.67 mm and a gap of up to 3.3 mm Citation[22]. To date, no experimental data exist concerning the ankle joint. The articular cartilage of the ankle joint is thinner than that of the knee Citation[36]; however, for the purpose of the study, comparable potentials for compensation of joint incongruities were assumed. Geometrical outcome analysis of the joint surface is mandatory for objective measurement of the result of the arthroplasty; however, an ideal instrument for outcome measurement needs to assess all incongruities present in a region of interest (ROI) (). Recently, an algorithm was developed to quantify the quality of a joint surface independently of the radius of the joint and the localization of the defect Citation[15]. The algorithm consists of five steps: 1) extraction of characteristic contours within an ROI on a 3D model of a joint (); 2) application of a central projection to the characteristic contours; 3) application of a digital filter to generate error signals for each characteristic contour (); 4) calculation of an error value by accumulating the error signals; and 5) mapping of the error score. The error score is transformed to a score with a standard range of 0 to 100 points describing the quality of the joint surface (0: ideal joint surface; 50: maximum step 1.67 mm, maximum gap 3.3 mm; and 100: prohibitive joint surface). shows the CAS-implanted talar graft of a trial operation with a posterior gap corresponding to an arthroplasty score of 89 points. We did not use the technique to evaluate the geometrical outcome of our cadaver series because it is not yet routinely applicable. It is currently used as a 2D technique, though in principle it can be applied three-dimensionally. Three-dimensional geometrical surface analysis would be of great value for objective outcome evaluation of articular surfaces and all kinds of reconstructive procedures that can be visualized volumetrically (e.g., osteosynthesis). This information may also lead to a better understanding of the value of joint incongruity or malreduction Citation[21].
Figure 7. Two-dimensional surface scanning within a region of interest and derivation of the arthroplasty score.
A century has passed since Lexer Citation[37] and colleagues performed the first homologous joint transplant. Orthopaedic surgeons since that time have shared the vision of biological joint replacement. The intention of our research was to contribute a module for achieving this common goal within a multidisciplinary approach.
Acknowledgments
The study was supported by the AO Research Fund (AO Foundation, Davos, Switzerland). None of the authors has professional or financial affiliations that may be perceived to have biased the study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Eingartner C, Heigele T, Dieter J, Winter E, Weise K. Long-term results with the BiCONTACT system–aspects to investigate and to learn from. Int Orthop 2003; 27(Suppl 1)S11–S15
- Langlais F, Belot N, Ropars M, Lambotte JC, Thomazeau H. The long-term results of press-fit cemented stems in total knee prostheses. J Bone Joint Surg Br 2006; 88(8)1022–1026
- Anderson T, Montgomery F, Carlsson A. Uncemented STAR total ankle prostheses. Three to eight-year follow-up of fifty-one consecutive ankles. J Bone Joint Surg Am 2003; 85-A(7)1321–1329
- Sailer R, Hackl W, Klestil T, Horbst W, Rangger C, Blauth M. Total endoprosthesis of the upper ankle joint after post-traumatic arthrosis. Unfallchirurg 2002; 105(2)170–173
- Wulker N, Flamme CH, Muller A, Wirth CJ. 10 years follow-up of arthrodeses of the hindfoot joints and upper ankle joint. Z Orthop Ihre Grenzgeb 1997; 135(6)509–515
- Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: A first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma 2006; 20(10)739–744
- Gobbi A, Francisco RA, Lubowitz JH, Allegra F, Canata G. Osteochondral lesions of the talus: Randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation. Arthroscopy 2006; 22(10)1085–1092
- Jarde O, Trinquier-Lautard JL, Garate F, de Lestang M, Vives P. Osteochondral lesions of the talar dome: Surgical treatment in a series of 30 cases. Rev Chir Orthop Reparatrice Appar Mot 2000; 86(6)608–615
- Rosenberger RE, Fink C, Bale RJ, El Attal R, Muhlbacher R, Hoser C. Computer-assisted minimally invasive treatment of osteochondrosis dissecans of the talus. Oper Orthop Traumatol 2006; 18(4)300–316
- Schottle PB, Oettl GM, Agneskirchner JD, Imhoff AB. Operative therapy of osteochondral lesions of the talus with autologous cartilage-bone transplantation. Orthopade 2001; 30(1)53–58
- Baums MH, Heidrich G, Schultz W, Steckel H, Kahl E, Klinger HM. Autologous chondrocyte transplantation for treating cartilage defects of the talus. J Bone Joint Surg Am 2006; 88(2)303–308
- Sijbrandij ES, van Gils AP, Louwerens JW, de Lange EE. Posttraumatic subchondral bone contusions and fractures of the talotibial joint: Occurrence of “kissing” lesions. AJR Am J Roentgenol 2000; 175(6)1707–1710
- Schaefer D, Martin I, Jundt G, Seidel J, Heberer M, Grodzinsky A, Bergin I, Vunjak-Novakovic G, Freed LE. Tissue-engineered composites for the repair of large osteochondral defects. Arthritis Rheum 2002; 46(9)2524–2534
- Shao X, Goh JC, Hutmacher DW, Lee EH, Zigang G. Repair of large articular osteochondral defects using hybrid scaffolds and bone marrow-derived mesenchymal stem cells in a rabbit model. Tissue Eng 2006; 12(6)1539–1551
- Sidler R, Gonzalez Ballester MA, Bonel HM, Styner M, Bardyn T, Nolte LP, Sudkamp NP, Kostler W. Computer-assisted arthroplasty using bioengineered autografts. IEEE Eng Med Biol Mag 2006; 25(4)63–69
- Haberland N, Ebmeier K, Grunewald JP, Hliscs R, Kalff RL. Incorporation of intraoperative computerized tomography in a newly developed spinal navigation technique. Comput Aided Surg 2000; 5(1)18–27
- Langlotz F, Stucki M, Bachler R, Scheer C, Ganz R, Berlemann U, Nolte LP. The first twelve cases of computer assisted periacetabular osteotomy. Comput Aided Surg 1997; 2(6)317–326
- Matziolis G, Krocker D, Weiss U, Tohtz S, Perka C. A prospective, randomized study of computer-assisted and conventional total knee arthroplasty. Three-dimensional evaluation of implant alignment and rotation. J Bone Joint Surg Am 2007; 89(2)236–243
- Sidler R, Kostler W, Bardyn T, Styner MA, Sudkamp N, Nolte L, Gonzalez Ballester MA (2005) Computer-assisted ankle joint arthroplasty using bio-engineered autografts. Proceedings of the 8th International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI 2005), Springs, CA, October, 2005, JS Duncan, G Gerig. Lecture Notes in Computer Science 3749, Springer, Berlin, 474–481, Part I.
- Brown TD, Rudert MJ, Grosland NM. New methods for assessing cartilage contact stress after articular fracture. Clin Orthop Relat Res 2004:, 423: 52–58
- Hahn DM. Current principles of treatment in the clinical practice of articular fractures. Clin Orthop Relat Res 2004:, 423: 27–32
- Huber-Betzer H, Brown TD, Mattheck C. Some effects of global joint morphology on local stress aberrations near imprecisely reduced intra-articular fractures. J Biomech 1990; 23(8)811–822
- McKinley TO, Rudert MJ, Koos DC, Pedersen DR, Baer TE, Tochigi Y, Brown TD. Contact stress transients during functional loading of ankle stepoff incongruities. J Biomech 2006; 39(4)617–626
- Sugano N, Sasama T, Sato Y, Nakajima Y, Nishii T, Yonenobu K, Tamura S, Ochi T. Accuracy evaluation of surface-based registration methods in a computer navigation system for hip surgery performed through a posterolateral approach. Comput Aided Surg 2001; 6(4)195–203
- Tamura Y, Sugano N, Sasama T, Sato Y, Tamura S, Yonenobu K, Yoshikawa H, Ochi T. Surface-based registration accuracy of CT-based image-guided spine surgery. Eur Spine J 2005; 14(3)291–297
- Woerdeman PA, Willems PW, Noordmans HJ, Tulleken CA, van der Sprenkel JW. Application accuracy in frameless image-guided neurosurgery: A comparison study of three patient-to-image registration methods. J Neurosurg 2007; 106(6)1012–1016
- Kendoff D, Bogojevic A, Citak M, Maier C, Maier G, Krettek C, Hufner T. Experimental validation of noninvasive referencing in navigated procedures on long bones. J Orthop Res 2007; 25(2)201–207
- van de Kraats EB, van Walsum T, Kendrick L, Noordhoek NJ, Niessen WJ. Accuracy evaluation of direct navigation with an isocentric 3D rotational X-ray system. Med Image Anal 2006; 10(2)113–124
- Potter HG, Linklater JM, Allen AA, Hannafin JA, Haas SB. Magnetic resonance imaging of articular cartilage in the knee. An evaluation with use of fast-spin-echo imaging. J Bone Joint Surg Am 1998; 80(9)1276–1284
- Carrino JA, Blum J, Parellada JA, Schweitzer ME, Morrison WB. MRI of bone marrow edema-like signal in the pathogenesis of subchondral cysts. Osteoarthritis Cartilage 2006; 14(10)1081–1085
- Ueblacker P, Wagner B, Kruger A, Vogt S, DeSantis G, Kennerknecht E, Brill T, Hillemanns M, Salzmann GM, Imhoff AB, Plank C, Gansbacher B, Martinek V. Inducible nonviral gene expression in the treatment of osteochondral defects. Osteoarthritis Cartilage 2004; 12(9)711–719
- Lynn AK, Brooks RA, Bonfield W, Rushton N. Repair of defects in articular joints. Prospects for material-based solutions in tissue engineering. J Bone Joint Surg Br 2004; 86(8)1093–1099
- McKinley TO, Rudert MJ, Koos DC, Tochigi Y, Baer TE, Brown TD. Pathomechanic determinants of posttraumatic arthritis. Clin Orthop Relat Res 2004:, 427 Suppl: S78–S88
- Tochigi Y, Rudert MJ, Saltzman CL, Amendola A, Brown TD. Contribution of articular surface geometry to ankle stabilization. J Bone Joint Surg Am 2006; 88(12)2704–2713
- McKinley TO, Rudert MJ, Tochigi Y, Pedersen DR, Koos DC, Baer TE, Brown TD. Incongruity-dependent changes of contact stress rates in human cadaveric ankles. J Orthop Trauma 2006; 20(10)732–738
- Millington SA, Li B, Tang J, Trattnig S, Crandall JR, Hurwitz SR, Acton ST. Quantitative and topographical evaluation of ankle articular cartilage using high resolution MRI. J Orthop Res 2007; 25(2)143–151
- The classic. Joint transplantation. By Eric Lexer, 1908. Clin Orthop Relat Res 1985; 197: 4–10