Abstract
The possibility of automatically navigating untethered microdevices or future nanorobots to conduct target endovascular interventions has been demonstrated by our group with the computer-controlled displacement of a magnetic sphere along a pre-planned path inside the carotid artery of a living swine. However, although the feasibility of propelling, tracking and performing real-time closed-loop control of an untethered ferromagnetic object inside a living animal model with a relatively close similarity to human anatomical conditions has been validated using a standard clinical Magnetic Resonance Imaging (MRI) system, little information has been published so far concerning the medical and technical protocol used. In fact, such a protocol developed within technological and physiological constraints was a key element in the success of the experiment. More precisely, special software modules were developed within the MRI software environment to offer an effective tool for experimenters interested in conducting such novel interventions. These additional software modules were also designed to assist an interventional radiologist in all critical real-time aspects that are executed at a speed beyond human capability, and include tracking, propulsion, event timing and closed-loop position control. These real-time tasks were necessary to avoid a loss of navigation control that could result in serious injury to the patient. Here, additional simulation and experimental results for microdevices designed to be targeted more towards the microvasculature have also been considered in the identification, validation and description of a specific sequence of events defining a new computer-assisted interventional protocol that provides the framework for future target interventions conducted in humans.
Introduction
Combining the principle of induced propulsion force on a ferromagnetic core from magnetic gradients with closed-loop control can lead to accurate displacement of an untethered object along pre-planned paths. In its simplest form, this object can be a simple untethered ferromagnetic bead like the one used to validate the protocol described here. However, more sophisticated versions may take the form of microparticles synthesized to take advantage of recent discoveries in nanotechnology and nanomedicine, or more advanced alternatives such as microdevices integrating more complex functionalities. Such untethered micro-objects, coupled with the possibility of accurate displacement along pre-planned paths in the vascular network, could provide new medical diagnostic and interventional tools capable of reaching regions in the human body which are currently inaccessible to catheterization. Such a paradigm, in which direct targeting could be performed in the vascular network by navigating untethered micro-objects and microdevices (including future intelligent components such as micro- and nanorobots) along pre-planned paths towards specific targets using new interventional platforms, could lead not only to innovative therapeutic and diagnostic applications in the human body, but also to an improvement in the efficacy of many existing medical interventions. In particular, oncology may benefit from such a new concept of direct targeting with special magnetic microparticles, referred to here as microcarriers, whereby secondary toxicity resulting from interventions such as chemotherapy and chemo-embolization could in many cases be minimized by preventing, or at least significantly reducing, the circulation of toxic agents through the systemic blood networks while enhancing therapeutic efficacy with lower toxic dosages.
The possibility of controlling microparticles for medical applications is not new. In 1965, aneurysm embolization studies were based on powders formed by iron microparticles spatially confined within magnetic-tipped catheters Citation[1]. Since then, other applications have been considered, including magnetic drug delivery with carriers that have evolved into state-of-the-art nanoparticles such as stealth magnetoliposomes or smart polymer-based magnetic particles Citation[2–5]. In parallel, state-of-the-art methods for targeting these particles have evolved. At present, these methods rely on external magnets or magnetized needles or catheters; however, because a magnet cannot be located within a target such as a tumoral lesion, targeting efficacy is reduced, with magnetic microparticles or microcarriers being attracted towards the same magnet (and hence away from the tumor) where the magnetic field intensity is stronger. Furthermore, targeting efficacy using this approach is expected to be reduced significantly for targets located deeper in the human body, where the distance between such a magnet and the tumor is likely to increase. As these previous methods rely on trapping the magnetic microparticles or microcarriers without navigation in the form of trajectory control over pre-planned paths, targeting efficacy would be reduced still further by the distance between the release site and the tumor, which can be quite significant in most cases, considering the actual reachable limits of catheterization. Even a relatively small distance separating the tumor from the release site could result in a significant loss of these microparticles in the systemic circulation due to complex microvasculature networks and related unpredictable micro-flows neighboring the tumor. Hence, magnetic targeting would greatly benefit from an increased role for computerized platforms in order to achieve automatic control over the spatial distribution and displacement of such microparticles along pre-planned trajectories.
Stereotaxis, Inc. (Saint Louis, MO; www.stereotaxis.com), for example, has proposed a computerized steering platform for catheters. Their magnetic-tipped catheters can be steered using actuated external magnets. The orientation of the catheter is controlled by computers with special infrastructures acting on the tilt angle of the external magnets. Proper corrective action is computed based on tracking information from X-ray projection images, but the system and the approach used cannot control the displacement of untethered objects as needed to accomplish accurate direct targeting in the human body, and their application has therefore been limited so far to computer-controlled catheter deflections during minimally invasive interventions.
Yesin et al. Citation[6], Citation[7] navigated magnetic microdevices in vitro using a custom-built apparatus that relies on Helmholtz and Maxwell pairs of coils where optical tracking is used. These microdevices have been developed for future surgical procedures in transparent media such as the vitreous humor, where direct line of sight using an optical microscope can be achieved. Because this approach uses optical tracking, it can only be considered for a few medical interventions where line of sight is possible, and cannot be used in endovascular applications to record the information needed by an external controller to compute the corrective actions necessary to maintain progress of such untethered microdevices along a pre-planned path.
The new computerized platform proposed here can provide an efficient interventional infrastructure for many medical applications in the human endovascular network, and particularly for direct tumor targeting. This platform not only provides a method for manipulating magnetic micro-objects such as microdevices including magnetic microcarriers in a 3D volume, but, more importantly, it integrates into the closed-loop navigation control MRI for feeding tracking information to an external controller, allowing effective displacements along pre-planned paths from the catheterization boundaries to specific targets.
In addition to their being widely available in hospitals, we showed that MRI scanners provide a magnetic actuation method without depth limitation Citation[8], Citation[9] while retaining the known advantages of X-ray-free unparalleled soft tissue contrast combined with high precision and sensitivity tracking Citation[10]. With these positive features linked to a special real-time interventional software architecture Citation[11], an MRI system can become a novel interventional platform for direct target interventions, as demonstrated previously by our group in an in vivo study Citation[12].
This paper aims not only at describing the computer-assisted protocol that was involved in achieving such interventions, but also at identifying some of the expansion modules needed for such a protocol, based on new results obtained with smaller micro-objects such as microdevices designed to reach deeper regions in the microvasculature. In particular, the paper describes a sequence of events defining a new protocol for successfully conducting such interventions in which medical and new technological procedures are seamlessly integrated and synchronized. More specifically, we show that such a protocol plays a crucial role in the success of these interventions and must therefore be developed to minimize changes in existing medical protocols while taking advantage of the added benefit of such an interventional platform, but always within interventional, physiological and technological constraints.
The paper first introduces the method of propulsion of such untethered micro-objects; the word microdevice will be used to include all possible types of navigable single untethered magnetic micro-objects up to and including a single agglomeration of such micro-objects designed to conduct a specific task. Emphasis is also placed on the advantages and limitations of this approach, followed by a description of the system architecture capable of implementing such a method. Next, the medical and technical protocol is described, including the animal or patient preparation when the protocol is described with a human subject in mind, and other preparatory issues such as trajectory planning. The protocol used during real-time in vivo navigation is then described. Some possible expansions of the protocol required for other types of applications, such as the use of navigable biosensors, are also discussed, along with some of the main challenges and issues to be considered when attempting to reach the microvasculature.
The fundamental principle and its main limitations
Although the proposed method may lead to enhanced targeting efficacy compared to other proposed approaches, it has limitations which must be considered during the execution of the protocol. Without a good understanding of the fundamental principle behind this technology and its limitations during an intervention, serious injuries or other consequences resulting from the loss of the untethered magnetic microdevice in the vascular network could occur. Such constraints are integrated into a software platform developed to help the medical staff guarantee a proper and safe execution of the interventional protocol and to prevent any loss of control.
Fundamentally, the magnetic force Fmag (N) acting on a magnetized particle is proportional to its magnetization M (A/m) and to the gradient of the magnetic field B (T), as shown in Equation 1:
The magnetization of the magnetic material M is a function of the ambient magnetic field, and reaches a maximum value or saturation magnetization (Msat) when this ambient magnetic field is sufficiently high, as is the case when placed in the tunnel of a conventional clinical MRI system where a DC magnetic field magnitude of at least 1.5 T is present. Since the same DC magnetic field will maintain the original orientation of a ferromagnetic microdevice when navigated in different directions through the vascular network, a spherical shape is typically considered to maintain a drag force independent of the direction of motion while eliminating the risk of tissue damage when moving in the blood vessels.
Despite the use of a material that reaches a high saturation magnetization level when placed in the bore of the MRI system to achieve maximum induced propulsion force, safe navigation without any complementary procedures in the protocol, as described later, can only be supported for relatively large ferromagnetic microdevices operating in larger-diameter vessels. This is due to the relatively low directional magnetic gradient magnitudes of approximately 40 mT/m provided by the three orthogonal coils in modern MRI scanners as presently used for MR-image encoding. Hence, higher gradients beyond the capability of existing MRI scanners will be required to act on microdevices designed to reach targets closer to the microvasculature. This limitation of modern scanners for this method is depicted in Equation 1. In order to be able to propel magnetic microdevices in the arterial network, scaling laws of magnetic propulsion require that special gradient coils be developed. An order-of-magnitude increase in gradient amplitude, compared to standard imaging gradients, is thus required. Although it is feasible to develop such enhanced gradient coils within actual technological constraints while operating within FDA recommendations, their implementation is likely to lead to a cost increase for the MRI hardware that will have to be counterbalanced by a significant improvement in treatment. Nonetheless, it is interesting to note that it is possible to reach locations in the vascular networks beyond current catheterization limits in modern MRI scanners without the need for additional hardware upgrades, especially if a complementary medical technique such as the use of a balloon catheter to control and reduce blood velocity in larger vessels is integrated as part of the interventional protocol. In addition to knowledge of physiological parameters such as blood velocities and pulsatile flow frequencies, parameters as defined in the variables in Equation 1 must also be accounted for and included in the software environment aimed at assisting the medical staff in the planning and execution of the interventional protocol, with particular attention being given to the scaling effects when smaller microdevices are being navigated.
Equation 1, for instance, shows that the magnetic force scales as the volume V of a spherical microdevice or the cube of its radius r. The weight W and buoyancy B of the microdevice are
The densities ρmat and ρfluid apply to the magnetic material and the fluid, respectively. The gravitational acceleration is represented by g. Both buoyancy and gravitational forces, being proportional to r3, will scale like the magnetic force as the size of the controlled microdevice decreases. In other words, the same magnetic gradient will cause the same acceleration on a smaller microdevice against the gravitational force, the buoyancy, and all volumic forces as it would on a larger microdevice. Particularly for small microdevices, the viscous drag force D caused by a fluid (blood in our case) is given by Stokes’ law, and is computed as
In Equation 4, μ is the viscosity of the fluid and Vrel is the velocity of the microdevice with respect to the fluid. The viscous drag force scales down with the radius of the spherical microdevice. When approaching the micrometric scale, the viscous drag force becomes predominant compared to the volumic forces. Hence, as r becomes smaller towards the micrometric scale, r3 becomes even smaller. Therefore, to reach the same magnetophoretic velocity, a smaller microdevice must be subjected to a larger magnetic gradient than would a larger device. However, the overall sizes of these microdevices are also constrained by physiological parameters such as the diameter of the blood vessels used to reach the targets and the types of microdevices used for specific applications.
Main limitations of the approach and its impact on the medical and technical protocol
It is obvious that by adding functionality to the microdevices being navigated the effective volume of embedded magnetic material will be reduced accordingly, leading to a reduction of induced propulsion force from a given magnetic gradient amplitude. Even if other forces, such as gravitational force, preventing 3D navigation of larger ferromagnetic devices become much less predominant for much smaller microdevices, allowing 3D navigation to take place, the controlled displacements of smaller magnetic microdevices, especially those with overall dimensions of only a few micrometers, remain more challenging due to the non-linear scaling effects as explained earlier.
To provide an idea of the future of this approach in medical interventions, some potential applications that may benefit from this method are identified. Such applications will in turn demand additional software modules to support an extension of the fundamental protocol, which will also be affected by different physiological parameters (e.g., blood flow, distance between vessel bifurcations, diameter of blood vessels, etc.) and different characteristics of the microdevices used (e.g., different magnetic material, different effective volume of magnetic material, etc.), and one must consider not only the type of target interventions that could benefit from this method, but also the characteristics of the associated microdevices.
We already know from previous theoretical and experimental results that it is possible to enhance medical interventions such as chemo-embolization where catheterization is a limiting factor, especially those involving magnetic particles or devices with diameters of a few hundred micrometers, in modern MRI scanners without any hardware upgrades. However, more demanding interventional procedures, especially those using smaller-diameter vessels to reach targets closer to the microvasculature, may require the use of additional gradient coils. This is particularly true when an intervention requires controlled navigation of microdevices to reach embolization sites which are closer to the tumoral lesions in order to achieve enhanced therapeutic efficacy.
As depicted in Equation 1, although the magnetic gradients must be increased, technological constraints limit the maximum amplitude for such additional coils to approximately 500 mT/m when designed for use with humans. The size of an adult human necessitates larger-diameter coils than those used for small animals, making adequate cooling of these coils within the space constraints of the bore of conventional clinical MRI platforms a real technological challenge. This cooling issue and related parameters must be integrated into the protocol to avoid an automatic shut-down of the system during an intervention. More precisely, the computer-assisted interventional protocol must take into consideration not only the maximum gradients that can be applied, but also the effective propulsion force that can be used and sustained during an intervention. Such effective propulsion force, which must be sufficient for safe endovascular navigation, is not only dependent on the propulsion duty cycle of the MR-imaging or propulsion coils in the case of a hardware-upgraded MRI platform, but also on the effective volume and saturation magnetization of the magnetic material embedded in the microdevices being navigated.
The propulsion duty cycle, corresponding to the percentage of time dedicated to propulsion within each navigation cycle, is an important yet critical parameter that is considered by the software modules assisting the medical staff during the intervention. During each navigation cycle, a period is typically dedicated to tracking the untethered microdevice while allowing sufficient time for the generation of 3D directional gradients for propulsion, with corrective trajectory control actions being computed in parallel from previous tracking data. Such navigation cycles must often be executed between 24 and 32 times per second, depending on physiological conditions including the level of perturbation in the blood vessels, in order to guarantee stability during closed-loop control so as to maintain the untethered microdevice within an acceptable error margin on a pre-planned path. However, particularly for the most demanding interventions where high magnetic propulsion gradients must be maintained, the coils tend to overheat, and the period of time dedicated to cooling and the corresponding cooling parameters must therefore be considered in the computerized interventional protocol, since this will translate into a decrease in effective propulsion force and must be predicted and managed in real time by the software platform.
The types of microcarriers or microdevices including microsensors used in specific applications will also affect the parameters considered by the computer-assisted protocol. For target chemo-embolization for example, the microdevices will typically take the form of biodegradable microparticles loaded with magnetic nanoparticles and anticancer drugs. The challenge here will be to load enough of these two components to achieve a good compromise between steering performance for targeting and therapeutic efficacy. Again, these design parameters must be entered in the computer-assisted protocol to guarantee successful and reliable target interventions.
The same holds true for microdevices such as micro-biosensors for applications such as target pH, glucose or CO2 measurements. Although several approaches are being investigated, one possible implementation could make use of specific polymer-coated magneto-elastic material (which would reduce the volume dedicated to magnetic material and hence the effective propulsion force level) for remote detection in an MRI environment. As an example, pH-sensitive polymers undergo alterations in their mechanical properties when placed in different pH environments: The polymer chains swell or shrink depending on the acidity of the medium into which they are placed, creating a stress modification in the material. Similarly, magneto-elastic material can strain in the presence of a magnetic field. By using an alternating magnetic field generated by the MRI RF coils, such a material can be forced into an oscillating state with a frequency related to the body's mass, density and Young's modulus. Hence, in the presence of an environmental change (change of pH, glucose or CO2), the polymer will induce a change in the device's density and mass distribution which in turn will lead to an oscillating frequency shift of the magneto-elastic material. Detection of such frequency shifts can be achieved using the same RF coils implemented in an MRI system. Preliminary work by other research groups also showed that standard coil antennas could be used to monitor the device's resonant frequency shift Citation[14]. The latter is one among many examples showing the possibility of expanding the range of applications of this technology by the implementation of navigable sensors, with a special MRI sequence being developed to monitor the frequency shifts of the microdevice and relate such frequency shifts to quantitative expressions Citation[15]. When linked to the platform, the protocol described in this paper can be adapted with sequences allowing resonant frequency-shift readings of future navigable micro-biosensors such as magneto-elastic beads coated with a pH-sensitive polymer, thus allowing precise diagnostics of physiological variations in patients. Again, however, the parameters of these microdevices would need to be implemented within additional software modules and linked to the computer-assisted interventional protocol described in this paper. This is just one example showing that the fundamental interventional protocol described here could be expanded to support a wider range of interventions, while the parameters of the software modules would be adjusted to the characteristics of their respective microdevices.
System overview
The interventional procedure described here has been validated in vivo using a standard clinical MRI system (Magnetom Avanto 1.5T, Siemens, Erlangen, Germany) with real-time feedback capabilities. Besides additional software modules specially developed for target medical interventions, no further upgrades have been implemented in the system, including the use of other tracking apparatus or additional propulsion gradient coils. A simple 1.5-mm ferromagnetic sphere was used to validate the protocol, providing well known and accurate specifications for use in the validation of our mathematical models while facilitating the tasks of evaluating the performance and limitations of the method and the related interventional protocol described here. To investigate and validate the protocol for use in future human interventions under real physiological conditions, the sphere was propelled in the carotid artery of a living swine through the automatic application of magnetic gradient pulses using the standard imaging magnetic gradient coils already installed in the clinical MRI system Citation[8], Citation[12]. A comprehensive custom software environment for the control, propulsion and tracking, which was also responsible for the accurate navigation of the sphere inside the swine Citation[10], was developed and validated with a feedback control frequency of 24 Hz. An overview of the real-time pulse sequence used and applicable to other similar interventions is illustrated in . The feedback navigation control was implemented between the reconstruction computer acting as a decision element and the MRI scanner acting as the execution element. Although this has proven to be a difficult task, the computer-assisted interventional protocol was implemented and linked to existing software in the MRI platform while maintaining sufficiently low latency for closed-loop control to achieve real-time performance at a level required for accurate navigation of untethered microdevices under real physiological conditions. To further validate the computer-assisted part of the protocol, additional experiments were also performed in more complex vasculature networks implemented in phantoms mimicking realistic human physiological conditions.
Figure 1. Overview of the computer architecture incorporated in the standard Siemens environment. The host computer is responsible for the real-time sequence compilation and initialization before it is sent for execution on the scanner. This computer also serves as the visualization computer after the image reconstruction step is over. The MRI scanner is the actual hardware that runs the pulse sequence for data acquisition. Finally, the reconstruction computer is responsible for the image generation after the data acquisition step on the scanner is completed. In the presented dedicated software environment, a feedback loop exists between the reconstruction computer and the MRI scanner which allows the pulse sequence to be modified on the fly.
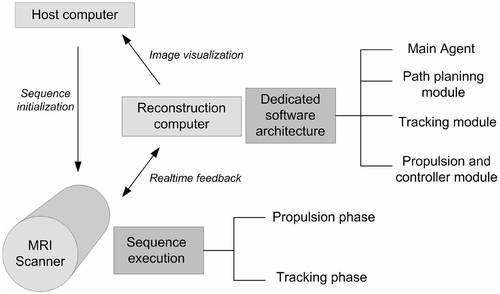
The real-time feedback capabilities also allow for the pulse sequences involved in such protocols, including RF pulses, magnetic gradients and ADC, to be modified as required on the fly. The dedicated software architecture designed to assist the execution of the interventional protocol is depicted in . It consists of a path-planning module, a tracking module, and a propulsion and controller module, with a central agent acting as a coordination and synchronization node for all the modules involved in the execution of the interventional protocol.
All these modules are located in a reconstruction routine in the reconstruction computer. A sequence environment is responsible for the application of the tracking sequence and the propulsion sequence which is executed by the scanner. The reconstruction routine is mainly responsible for command generation in order to propel the microdevice in a given direction based on its actual position and the pre-computed trajectory. Since the microdevice is moved using the magnetic gradient coils already present in the MRI system, the computed command calculated inside the propulsion and controller module is a magnetic gradient amplitude and direction to be applied in the next pulse-sequence propulsion phase. The real-time MRI pulse sequence developed is thus a successive repetition of a propulsion phase which consists of the application of a magnetic gradient oriented in space with given amplitudes and an off-resonance tracking phase within the real-time constraints of the applications. This tracking phase is based on a technique called MS-SET which has been developed by our group and is described in more detail in reference Citation[11]. An overview of the pulse sequence is depicted in .
Figure 2. Overview of the real-time sequence and image calculation processes for the navigation of the magnetic sphere. A sequence kernel composed of a real-time trigger event, a propulsion phase event, and a tracking event is repeated over time. The real-time trigger event starts the control module process for the command generation, and the tracking phase calls up the position module process for the device position calculation.
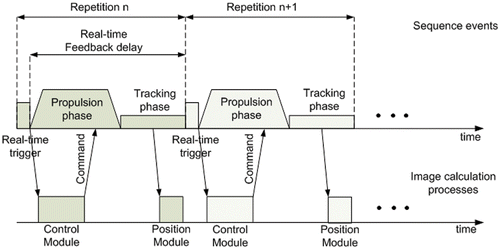
The image calculation environment consists of two processes running simultaneously. The first process contains the control module, which computes the required commands to be applied in the next propulsion phase of the real-time sequence. This process is called up through the real-time feedback loop routine located in the sequence described above. The second process is the tracking module and is called up during the tracking phase of the sequence. It is responsible for the device's position computation based on the acquired data from the tracking sequence.
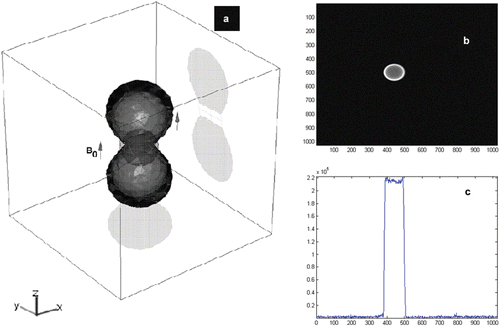
Projections taking advantage of the magnetic field being induced by the sphere (as depicted in ) or other untethered microdevices are used for tracking Citation[10]. Following the application of a non-selective off-resonance RF pulse, only the protons surrounding the device are excited. Readout gradients are then used to acquire three orthogonal projections that are processed by the tracking module. An example of an MR-image taken with the MS-SET method is depicted in , with the resulting projection depicted in .
Figure 3. (a) A 3D simulation of the excited volume and corresponding projection images with the MS-SET method. When this tracking method is used during the experiment, applied RF excitation signals are tuned to the equipotential magnetic curves generated by the magnetic signature of the sphere being tracked. The 3D position of the ferromagnetic object is obtained using a correlation function performed on each k-space line of each of the three axes corresponding to the three projections necessary to determine the best possible accurate localization of the sphere within the time constraint required to guarantee stability of the feedback controller. (b) MRI images of the ferromagnetic sphere with the MS-SET method in the transversal plane. (c) Projections of the images in (b) along the readout direction.
Once computed, the position is stored in the control module for the computation of the subsequent commands. As stated earlier, a central agent is responsible for the overall interactions between the modules and for the commands responsible for the running pulse sequence.
The real-time feedback delay depicted in is the minimum time allowed for the command computation and transmission, and is set by the user prior to the intervention. If the delay is any shorter, the command computation cannot be completed and the sequence is aborted. Since the sequence tracking phase duration is fixed and lasts 22 ms for a 3D positioning scheme, the propulsion phase delay is thus only dependent on the real-time feedback delay and is equal to the real-time feedback delay minus the tracking delay. A longer propulsion phase translates into a longer force application time on the microdevice, but also into a lower operating control frequency. Moreover, a longer propulsion phase means a longer contribution time for the magnetic gradient coils, which could lead to a heating threshold overshoot as described earlier, preventing the execution of the sequence.
Interventional protocol
The resulting protocol, which has been validated in vitro in phantoms mimicking complex human vasculatures and in vivo in animals approaching the human model using the same medical team, operating room and equipment that would be used for human patients, is descibed in this section. The specific protocol described here is for an intervention in the right carotid artery.
First, the patient (a 25-kg swine in our experiment) is put under general anesthesia (Pento-barbital). Next, a 6-F 80-cm introducer catheter (Cook Medical, Inc., Bloomington, IN) is inserted through a right femoral into the proximal portion of the right carotid artery under fluoroscopic guidance (HICOR/ACOM-TOP, Siemens, Erlangen, Germany). A short 5-F introducer is also positioned in the left femoral artery, and a 5 mm × 18 mm angioplasty balloon (AV100, Medtronic, Santa Rosa, CA) is advanced under fluoroscopic guidance in the distal portion of the right common carotid artery (10 cm downstream from the tip of the long introducer) over a 0.018-inch guidewire. The long introducer is used as the release route for the magnetic microdevice (or agglomeration of microdevices) to be controlled, whereas the optional balloon catheter is used to control the flow and eventually stop the blood flow for a short instant to facilitate retrieval of the microdevice, if required, upon completion of the intervention.
The patient is then placed in the MRI scanner and centered inside the MRI bore with respect to the ROI, in this case the right carotid artery. Body array coils are used to collect the MRI signals.
Then, since tracking of a ferromagnetic microdevice relies on its magnetic signature, it is critical to have no other magnetic sources in the vicinity of the imaging volume. Therefore, an off-resonance imaging sequence is first used to ensure that the volume of interest is free from magnetic perturbations that could interfere with the tracking phase. Any magnetic objects other than the propelled microdevice, such as a metallic tag used to identify the animal in our particular case or any other non-MRI-compatible objects (e.g., medical instruments for anesthesia) located in the vicinity of the imaging region, might cause perturbations that could confuse the automatic navigation control software. If perturbations do occur, the same imaging sequence can be used to locate the source.
Once the patient has been tested for magnetic perturbations, a roadmap of the environment and the trajectory for the microdevice are determined. The first phase of this process consists of imaging the region of interest (ROI) using a standard MRI angiographic sequence with gadoteridol (ProHance, Bracco Diagnostics, Mississauga, Ontario, Canada). Threshold filtering is applied on the 3D scan to allow better visualization of the targeted region before initiating the path-planning phase. To determine the path the microdevice will follow, waypoints are placed in the acquired volume (). The next step consists of transforming these points in the global MRI coordinate system or axis of reference. The registration software module developed from a combination of an MRI sequence and a Matlab script is used to analyze the position of the microdevice, transform the waypoint coordinates to the MRI frame of reference, and display the result over the image acquired earlier. The trajectory represented by waypoints contained in a file with a reference to the isocenter of the MRI is then performed Citation[13]. Once the waypoints are in the correct coordinate system, they are saved in a file containing all the 3D positions to be reached during the intervention. However, before initiating the navigation sequence, the list of waypoints is loaded into a memory space in the control module to allow fast access during the operation.
Figure 4. A 3D volume of the carotid artery of the swine filtered at 50% of the image scalar intensity range. Dots show the waypoints followed by the sphere; circles show the precision regions of radius φ. [Color version available online.]
![Figure 4. A 3D volume of the carotid artery of the swine filtered at 50% of the image scalar intensity range. Dots show the waypoints followed by the sphere; circles show the precision regions of radius φ. [Color version available online.]](/cms/asset/471d1e8e-ee80-4559-9bda-3231daf67b65/icsu_a_355295_f0004_b.gif)
The final preparation phase consists of a fine tuning of the imaging parameters. The microdevice is first attached to a catheter which is inserted into the artery and brought as close as possible to the operating region. The tracking sequence is then executed in order to fine-tune the parameters that depend on the magnetic characteristics and size of the microdevice being used, as well as on the background tissues where the acquisition is performed. The goal of the tuning process is to obtain the best SNR for tracking purposes. This is achieved by the acquisition of an image based on the same image timing and parameters as the tracking projections. The optimal parameters are the ones that give a dark background and a signal coming only from the medium in the device's neighborhood representing the excited spins. The parameters to be optimized are the offset frequency, the flip angle, and the dephaser gradients used to recover the signal lost through magnetic field inhomogeneity.
The 6-F inner dilator of the long introducer catheter is then used to push the untethered microdevice inside the long introducer. The microdevice is brought to a pre-release position 15 mm from the distal tip of the long introducer, using a color marking on the inner dilator as a visual landmark. A real-time imaging sequence provided by Siemens (Trufi-irttt) is used to monitor the introduction of the microdevice through the long introducer. The balloon catheter is also inflated to prevent the microdevice from being carried away in case of unplanned problems. The real-time MRI sequence is then started, and the microdevice can then be released by pushing the dilator all the way through the long introducer. Once the microdevice has been released, its movement is dealt with by the control sequence, which objective is to navigate the device by following the waypoints plotted on a special computer user interface to indicate the pre-planned path to be followed by the device until it reaches a specific target.
During the intervention, the microdevice position coordinates computed by the tracking module are compared with the next waypoint to be reached. If the distance between the device and the waypoint is contained within a circle of radius φ centered on the waypoint's position (), the waypoint is considered to be reached and the next waypoint is loaded as the next destination. Although the radius could be set to lower values for more accurate motions, it was set to φ = 10 mm in previous experiments that minimized navigational constraints while operating within acceptable trajectory error margins for this particular intervention and set of physiological parameters.
Real-time navigation of the ferromagnetic microdevice requires a proper real-time feedback delay to be chosen in order to allow both sufficient effective propulsion force and a sufficiently high operating frequency to ensure the stability and efficiency of the controller. Prior to conducting in vivo interventions, many navigation experiments in MRI phantoms were performed to obtain empirically the optimal real-time feedback delay. A feedback delay of 41 ms was chosen for the in vivo interventions conducted in the carotid artery, leading to a propulsion phase delay of 19 ms, considering a fixed tracking time of 22 ms. In this particular case, the total sequence repetition time is thus 41 ms, leading to an achievable operating frequency of 24 Hz for automatic closed-loop navigation control of a 1.5-mm spherical object moving at a maximum velocity of 13 cm/s. This operating frequency is adequate for the device's navigation through the selected trajectory described here, but could be changed when operating in other types of blood vessels and/or with different types of microdevices.
The main parts of the user interface, such as the MR images used for animal or patient localization, off-resonance sequences, and the real-time device's localization software, are depicted in . The MRI sequence controlling the device feeds data to the user interface section (item 6 in ). Every time the sequence acquires a new position, it records it in a text file located on the MRI computer. The user interface continuously parses that file looking for new data to be displayed. When new data is added to the file by the navigation sequence, the interface detects the change and updates the display to present the actual current state of the device trajectory, providing useful information during the intervention. The refresh rate of the display can be adjusted by the user to deliver better accuracy on the instant display. The text file can later be saved and re-used in order to replay the interventional data on the user interface.
Figure 5. User interface showing (1 and 2) the high-resolution MR images used to position the pig; (3) the off-resonance image used for the magnetic compatibility check and the tracking sequence calibration; (4 and 5) the Siemens sequence and protocol editor; and (6) our custom software showing the real-time position of the bead superimposed on a scale frame. [Color version available online.]
![Figure 5. User interface showing (1 and 2) the high-resolution MR images used to position the pig; (3) the off-resonance image used for the magnetic compatibility check and the tracking sequence calibration; (4 and 5) the Siemens sequence and protocol editor; and (6) our custom software showing the real-time position of the bead superimposed on a scale frame. [Color version available online.]](/cms/asset/8918eca2-e75c-4f08-8745-6842155674d6/icsu_a_355295_f0005_b.gif)
In some instances, especially when the overall dimensions of the microdevice are significant, retrieval may be required. During retrieval, the balloon catheter can be used to reduce or even temporarily stop the blood flow to ease the procedure. A permanent magnet at the tip of a catheter is then introduced into the artery and, once the catheter tip is stabilized, the microdevice is propelled towards the magnet. Once attached to the magnet, the microdevice is recovered by retrieval of the catheter. The real-time imaging sequence provided by Siemens (Trufi-irttt) is also used to monitor the extraction of the microdevice.
Short-term expansions of the interventional protocol
Among many possible expansions of the fundamental protocol described in this paper, those allowing target interventions more deeply towards the microvasculature, such as direct tumor targeting for enhanced chemotherapy and/or chemo-embolization, are most likely to be implemented initially. Such target medical interventions require an agglomeration of many microdevices with overall diameters ranging from a few micrometers to a few tens of micrometers. The main issues to be considered in the computer-assisted protocol described here are two-fold. First, a significant reduction of the effective volume of magnetic material in each microdevice will lead to a significant decrease in the magnetophoretic velocities. Second, unlike larger-diameter vessels, the blood vessels in the microvasculature are too small to be visualized with any medical imaging modality, including clinical MRI scanners. Therefore, the expanded protocol under development in our laboratory would make use of additional gradient coils operated in a time-multiplexed fashion, with the tracking sequences using specially developed software modules. Furthermore, the protocol dealing with path-planning in the microvasculature is expected to differ from that for larger-diameter blood vessels, such as the approach described here for the carotid artery using waypoints. The protocol will most likely consist of a series of several injections of an agglomeration of biocompatible polymeric microparticles loaded with magnetic nanoparticles. These initial microparticles will not contain toxic therapeutic agents so as to avoid secondary toxicity in the systemic circulation, since targeting efficacy during this initial phase is expected to be lower. Successive minimum doses of such microparticles will then be injected and steered using different directional patterns towards the tumoral lesion until a satisfactory sequence of directional gradients is found. Then, a dose of therapeutic agents loaded in similar microdevices will be injected and steered toward the tumor using the same steering or navigation sequence as previously validated. It should be noted that, in many instances, sufficient target therapeutic efficacy could be achieved, albeit less effectively than with target chemotherapy, by using larger microdevices containing more magnetic material coupled with target chemo-embolization. In this approach, finding a proper navigation path would be much easier, since the regions neighboring the tumor, rather than the tumor itself, can be targeted, thereby avoiding the use of much smaller microdevices containing less magnetic material and the need to navigate in the anarchic microvasculature networks stimulated by tumoral angiogenesis, where vessel diameters may be as small as 4–5 µm.
Summary and conclusion
A medical and technical protocol suitable for target endovascular interventions using a clinical MRI system has been described. The protocol integrates all the main components, such as propulsion, imaging, device tracking, and computer-based navigation control of an untethered microdevice, within a medical interventional environment and procedure. The protocol not only makes use of the enhanced features of such novel medical platforms based on MRI technology, but was also developed within the technological and physiological constraints identified in this paper. The protocol has been enhanced with specially developed software modules that have been integrated and linked with the existing software environment found in modern clinical MRI scanners. These enhancements include not only providing assistance when preparing such interventions, including path or trajectory planning, but also assisting medical staff in critical real-time sequences of operations. The latter include but are not limited to the generation of 3D propulsion gradients, tracking, and trajectory control that must be dealt with at frequencies higher than 24 Hz, which are beyond human capabilities.
The protocol described here has been successfully validated in living animal models with physiological parameters comparable to those found in humans, in an attempt to define a fundamental interventional protocol that could be used for future MRI-based target interventions in hospitals and clinics. The experiments confirmed proper synchronization and coordination between the medical and technical aspects involved in the interventional protocol. Although many interventions could be investigated, the navigation of a 1.5-mm ferromagnetic sphere in the carotid artery of a swine was used as a reference to provide an idea of a fundamental interventional protocol that could easily be adapted to humans, in which the interventional protocol would typically be carried out in a manner similar to that described in this paper. Nonetheless, further investigations and developments are required to expand and adapt the proposed protocol for assisting in the many future target interventions that could benefit from such technology. In particular, the use of smaller microdevices aimed at targeting the microvasculature will represent new challenges and contraints that the computer-assisted interventional protocol will have to deal with. However, the protocol as described will most likely serve as the framework and an initial proof of concept, as well as a reference for the procedures used to conduct such novel medical interventions.
References
- Alksne JF, Fingerhunt AG. Magnetically controlled metallic thrombosis of intracranial aneurysms. Bull Los Angeles Neurol Soc 1965; 30: 153–155
- Alexiou C, Arnold W, Hulin P, Klein RJ, Renz H, Parak FG, Bergemann C, Lubbe AS. Magnetic mitoxantrone nanoparticle detection by histology, X-ray and MRI after magnetic tumor targeting. J Magnetism Magnetic Materials 2001; 225(1–2)187–193
- Babincova M, Cicmanec P, Altanerova V, Altaner C, Babinec O. AC-magnetic field controlled drug release from magnetoliposomes: Design of a method for site-specific chemotherapy. Bioelectrochemistry 2002; 55(1–2)17–19
- Morales MA, Jain TK, Labhasetwar V, Leslie-Pelecky DL. Magnetic studies of iron oxide nanoparticles coated with oleic acid and Pluronic block copolymer. J Appl Phys 2005; 97(10)10Q905
- Viroonchatapan E, Ueno M, Sato H, Adachi I, Nagae H, Tazawa K, Horikoshi I. Preparation and characterization of dextran magnetite-incorporated thermosensitive liposomes: An on-line flow system for quantifying magnetic responsiveness. Pharm Res 1995; 12: 1176–1183
- Yesin KB, Exner P, Vollmers K, Nelson BJ. Design and control of in-vivo magnetic microrobots. Proceedings of the 8th International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI 2005), JS Duncan, G Gerig. Palm Springs, CA, October 2005. Part I. Lecture Notes in Computer Science 3749., Springer, Berlin 2005; 819–826
- Yesin KB, Vollmers K, Nelson BJ. Modeling and control of untethered biomicrorobots in a fluidic environment using electromagnetic fields. Int J Robotics Res 2006; 25(5–6)527–536
- Mathieu JB, Beaudoin G, Martel S. Method of propulsion of a ferromagnetic core in the cardiovascular system through magnetic gradients generated by an MRI system. IEEE Trans Biomed Eng 2006; 53(2)292–299
- Mathieu JB, Martel S. Magnetic steering of iron oxide microparticles using propulsion gradient coils in MRI. Proceedings of the 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS2006). New York, NY 30 August-3 September, 2006; 472–475
- Felfoul O, Mathieu JB, Beaudoin G, Martel S. MR-tracking based on magnetic signature selective excitation. IEEE Trans Med Imaging 2008; 27(1)28–35
- Chanu A, Aboussouan E, Tamaz S, Martel S. Sequence design and software environment for real-time navigation of a wireless ferromagnetic device using MRI system and single echo 3D tracking. Proceedings of the 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS2006). New York, NY 30 August-3 September, 2006; 1746–1749
- Martel S, Mathieu JB, Felfoul O, Chanu A, Aboussouan E, Tamaz S, Pouponneau P, Beaudoin G, Soulez G, Yahia LH, Mankiewicz M. Automatic navigation of an untethered device in the artery of a living animal using a conventional clinical magnetic resonance imaging system. Appl Phys Letters 2007; 90: 114105
- Aboussouan E, Martel S. High precision absolute positioning of medical instruments in MRI systems. Proceedings of the 28th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS2006). New York, NY 30 August-3 September, 2006; 743–746
- Cai Q, Zeng K, Ruan C, Desai TA, Grimes CA. A wireless, remote query glucose biosensor based on a pH-sensitive polymer. Analytical Chem 2004; 76: 4038–4043
- Chanu A, Martel S. MRI driven nano biosensor for wireless physiological data measurements using deformable polymers coated magnetoelastic devices. Proceedings of the 7th IEEE International Conference on Nanotechnology (IEEE-NANO2007). Hong KongChina August, 2007