Abstract
This paper describes a non-invasive remote temperature measurement technique integrated with a biomechatronic surgery system devised in our laboratory and named FUSBOT (Focal Ultrasound Surgery RoBOT). FUSBOTs use High-Intensity Focused Ultrasound (HIFU) for ablation of cancers/tumors and targets accessible through various soft-tissue acoustic windows in the human body. The focused ultrasound beam parameters are chosen so that biologically significant temperature rises are achieved only within the focal volume. In this paper, FUSBOTBS, a customized system for breast surgery, is taken as a representative example to demonstrate the implementation and the results of non-invasive feedback during ablation. An 8-axis PC-based controller controls various sub-sections of the system within a safe constrained work envelope.
Temperature is a prime target parameter in ablative procedures, and it is of paramount importance that means should be devised for its measurement and control in order to design optimal dose protocols and judge the efficacy of FUS systems. A customized sensory interface is devised and integrated with FUSBOTBS, and dedicated software algorithms are embedded for surgical planning based on real-time guidance and feedback. Variations in the physical parameters of the tissue interacting with the incident modality are used as surgical feedback. The use of real-time ultrasound imaging and data processed from various sensors to deduce lesion position and thermal feedback during surgery, as integrated with the robotic system for online surgical planning, is described. Dynamic registration algorithms are developed for compensation and re-registration of the robotic end-effector with respect to the target, and representative empirical outcomes for lesion tracking and online temperature estimation in various biological tissues are presented.
Introduction
Background
High-Intensity Focused Ultrasound (HIFU) has emerged as a non-invasive surgical modality for tissue ablation in deep-lying targets in soft tissues and organs. Controlled HIFU exposure results in thermal coagulation of tissues at localized target sites. The region of intended damage is called a lesion, where temperatures on the order of 60–80°C are reached. Dosage parameters such as input power levels up to 300 W and exposure duration of 1–10 seconds have been reported in the literature Citation[1–4]. Tissue ablation with HIFU is predominantly achieved by the conversion of the mechanical energy of an ultrasound wave into heat energy in the focal region of the transducer. The surgical potential of ultrasound was recognized in the 1950s, and it was initially used mainly for treating neurosurgical and neurological abnormalities. Recently, the advent of precise imaging modalities and advances in transducer design have stimulated research in Focused Ultrasound Surgery (FUS) and have attracted further clinical attention. Experimental studies and clinical data are available for various tissue types from several laboratories worldwide and have shown promising results. The techniques and strategies adopted for applying this modality and for facilitating effective energy transfer in the desired target differ for various tissues and organs. The size, shape and placement of the lesion primarily depend on the characteristics of the HIFU transducers, penetration depth, targeting efficiency (while scanning over the region of interest) and intervening tissue, as well as on the tissue-modality interaction. While FUS is being actively evaluated for efficacy in pre-clinical trials, some HIFU-based commercial devices are also available, including Sonablate (Focus Surgery, Inc., Indianapolis, IN), Ablatherm (EDAP, Lyon, France), ExAblate (InSightec Ltd., Tirat Carmel, Israel), and the Haifu JC system (Chongqing (HAIFU) HIFU Technology Co. Ltd., China).
Surgical feedback for ablation: present state of the art
Despite the great potential of HIFU as a non-invasive surgical tool for cancer management, and the wealth of experience obtained using single, multiple and phased array-based HIFU transducers (in either extracorporeal or intra-cavitary modes) in animal studies, its use in the clinic is still very limited. One reason for this could be the slow progress in the design and practical implementation of non-invasive surgical assessment and control procedures. Real-time guidance, feedback and control are highly desirable for making the system safe and clinically applicable. Surgical feedback during ablation specifically requires that temperature elevations in the treatment volume be monitored and controlled in an appropriate non-invasive manner. Among the techniques being investigated and developed to provide non-invasive temperature feedback are MRI, tomography and microwave radiometry. Each of these techniques has its own limitations and advantages.
MRI guidance and MRTI (Magnetic Resonance Thermal Imaging) techniques have been developed for thermal monitoring using changes in relaxation time and temperature-dependent water proton-resonance frequency shift Citation[5–8]. The entire HIFU application system must be accommodated in an operating table that can be slid inside the closed magnet assembly of a standard MRI machine, allowing interventional temperature shots to be taken for the tissue under investigation. Various successful applications have been reported in breast, uterine fibroids, liver, etc. in several clinical settings. However, integration of HIFU with an MRI system, besides bringing high cost to the treatment procedure, also involves overcoming the problem of compatibility of the ultrasound source with high magnetic fields. Though MRI has the advantage of better image quality and the ability to monitor temperature, ultrasound-based techniques are viewed more favorably as they can be easily integrated into HIFU systems, are portable, and have relatively lower costs.
In recent years, there have been significant advances in the imaging capability of medical ultrasound devices. With improved computing power and signal processing techniques, the viability of using diagnostic ultrasound-based imaging devices capable of visualizing the minute details of tissues and features of the human anatomy in real time has been extensively explored. This possibility has paved the way for ultrasonic imaging devices to be combined with FUS systems to detect the localized sites for surgical guidance. Ultrasound techniques under investigation for non-invasive feedback have revolved around tissue characterization parameters such as the attenuation coefficient, speed of sound, backscattering coefficient, and elasticity, etc. Citation[9–16].
The temperature dependence of ultrasound parameters such as speed of sound and attenuation in the target tissue has previously been used as a non-invasive feedback parameter for ultrasonic hyperthermia Citation[10–11]. Using a microprocessor-based system, empirical relationships between temperature, intensity and velocity among different samples were correlated, and it was suggested that the temperature inside a tissue could be estimated by interpolating the non-invasively measured values of velocity at various exposure levels.
An analytical model proposed by Maass-Moreno et al. was used to relate time shifts to changes in temperature distribution Citation[12]. The model showed that the echo shifts depend mostly on changes in the mean velocity along the acoustic path. However, a severe shortcoming of this study was the dependence on the presence of two or more stationary reflectors in the tissue, a condition that is not easily achievable clinically. Seip and Ebbini Citation[13] proposed the measurement of local frequency shifts using backscattered RF data as a function of time. The backscattered ultrasound pulse-echo data were acquired by the imaging transducers as a function of time while the tissue samples were heated and cooled. A bare-wire thermocouple was used to measure the tissue temperature at the heating location. For non-invasive assessment, temperature and frequency shifts were correlated.
In a study by Bailey et al. Citation[14], diagnostic ultrasound was used to target and monitor real-time focused ultrasound therapy. In this system, a HIFU transducer and several US scan heads with different frequencies were placed with the focus in the imaging plane. The HIFU and ultrasound were synchronized to relegate the interference to the image fringe. It was found that the HIFU transducers produced a localized hyperechoic region visible on the B-mode image. In another study by Makin et al. Citation[15], B-scan images for lesion monitoring using miniaturized dual-functionality ultrasound arrays (to image the treatment regions in real time and treat the target regions) were investigated in excised porcine liver tissue.
It is known that protein denaturation induces changes in the elastic modulus of proteins, thus enabling variation in the elastic modulus to be tracked for feedback purposes. Speckle noise is directly related to ultrasonic scattering from tissue microstructure, and can thus serve for localizing and monitoring lesions. Studies on the stress-strain behavior of collagenous tissue undergoing different degrees of prior thermal damage were conducted. These studies showed that the heat-induced changes in the mechanical properties are only related to the extent of the prior damage and do not depend on the specific thermo-mechanical history that caused the damage Citation[16]. The strain contrast of the lesion and the background was found to be visible during ablation, but quantitative and online estimates of the temperature were not demonstrated.
The temperature feedback during FUS is pivotal for localizing and monitoring lesions in target sites. Precise targeting using optimum energy protocols for a given tissue type and feedback on lesion position and temperature during the exposure are crucial for the safety and success of clinical procedures. Establishing and quantifying temperature increases in the tissue with the above-mentioned techniques usually requires invasive means such as the insertion of thermocouples into the focal region. However, the spot measured by an invasive thermocouple does not always yield the actual temperature rise due to the perturbations caused by thermocouples owing to thermal conduction.
In our present research, we attempt to make use of propagation speed, backscattered data and time/phase shift for interpreting and displaying the location of lesions, as well as for quantifying the local temperature increase during surgery, by developing an integrated sensory suite and robotic system (multi-sensor data acquisition, information fusion and online planning and display) for registration with the ablation suite.
Methodology and empirical set-up
Ultrasound beams can be focused at a desired distance from the source in the frequency range used for medical applications (0.5–15 MHz). The biological results of ultrasonic irradiation depend upon the delivered dosage, the threshold of irreversible damage of the normal tissue, and the temporal aspects of any relevant homeostatic mechanisms. Heat dose delivery is decided in the treatment-planning phase. The temperature in the insonified tissue will depend upon the exposure conditions, which are governed by various factors such as the ultrasound power, transducer geometry, frequency, spatial and temporal distribution of the field, exposure time, absorption properties of the tissue, attenuation in the intervening tissue, acoustic reflection and refraction, and blood perfusion rate, etc. Citation[17–19]. Temperatures thus produced cause rapid, complete and irreversible damage to the exposed tissue. Stress mechanisms, such as cavitation, should be avoided due to their unpredictability.
In the non-invasive approach, the acoustic energy is applied completely externally with no access incisions. The common type of applicators in this category are spherical focused transducers (focused bowls) with large apertures (on the order of 8–10 cm) so as to withstand and transmit sufficient energy to remote sites of interest. These extracorporeal transducers can be used to treat/ablate tissues which can be approached through large acoustical windows via coupling through appropriate couplant liquid, e.g., degassed water. However, various factors require attention in order to attain efficacious results from sonication of biological tissues. The specific location of the tissue or organ in the human body also affects the design of the applicators. Based upon the dimensions, location and nature of the abnormality, the size and shape of the applicators can be evaluated by modeling the ultrasonic field in front of the transducers.
Multi-probe approach for FUS
In conventional procedures, the HIFU beam is generated using large focused spherical bowl transducers so as to achieve a desirable energy level in the focal zone. Thermal damage in intervening tissue resulting from off-focus hot spots may also occur with such approaches due to energy dispersion in the secondary lobes. To avoid such unwanted tissue damage, it is necessary to control the desired thermal dosage within the focused region of the HIFU beam. A technique using multiple probes for HIFU-based neurosurgery was previously devised Citation[17–18] as an alternative solution to minimizing the effects of off-focal hot spots. In this approach, instead of using a large single probe, it was proposed to use small multiple probes working in unison so as to obtain constructive interference in the desired target (as shown in ). Flexibility in controlling intensity levels in the target can be achieved by using multiple probes to simultaneously ablate the target at different angles. The methods presented in this paper use such a multi-probe technique for field distribution in selected targets.
Figure 1. Schematic of multi-probe approach (left) and multi-probes arranged on a robotic end-effector (right). (Three probes are shown as an example: the actual number may vary.)
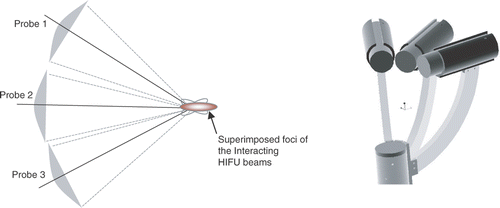
Numerical models were contrived to discern axially symmetric calculations for beam patterns using diffraction analysis and Huygens’ principle for focused bowl geometries. These models help in the predictive analysis of the intensity distribution in a given tissue of interest. Having selected the required field parameters and transducer specifications, and the field distribution in two and three dimensions, it is possible to study the location and dimensions of the focal region. For further details on modeling algorithms, please refer to references Citation[17–19]. The simulation results were verified using a customized mechatronic testjig developed in our laboratory Citation[20–21].
Thermal mechanisms
The rise in temperature in the irradiated region depends upon various exposure parameters. After deciding the transducer specifications, and thus the wave characteristics, by using the models as explained earlier, and using the data available for attenuation in a given tissue, the rise in temperature in a specified tissue remains a function of intensity, time of irradiation, tissue attenuation and perfusion characteristics. Temperature evaluation incorporating attenuation effects on intensity levels in a given medium, while assuming blood perfusion is insignificant in short exposures, can be evaluated by solving Penne's bio-heat equation Citation[22]:where T is the tissue base temperature, Ta the local arterial blood temperature, Tb the local venous temperature, cb the specific heat of blood, c the specific heat of the tissue, ρ the tissue density, ω the blood perfusion rate, q the volumetric heat generation rate, and κ the thermal conductivity of the tissue. The effects of blood perfusion during short ablation periods are assumed to be negligible (ω = 0). With this assumption, the temperature elevation in the exposed area was modeled using a multi-probe approach Citation[17–19].
Empirical set-up and user interface
The ablation feedback and dynamic registration algorithms are integrated in custom-built robotic systems named FUSBOTs (Focal Ultrasound Surgery RoBOTs), which are designed for various applications such as breast surgery, urological applications, and neurosurgical applications (differentiated with the superscripts BS, US and NS, respectively). The clinical FUSBOTBS for breast applications is taken as a representative example for monitoring the treatment procedure and for the results presented in this paper. It has four degrees of freedom (DOFs) for localizing multiple HIFU transducers to a target site, and has a cylindrical work envelope: vertical axis (VA), horizontal axis (HA), rotation axis (RA) and orientation axis (OA). The experimental set-up for real-time monitoring is shown in . Using interchangeable end-effectors with additional jig axes (not shown in the figure), this operating system can be extended to enable trans-abdominal reach. The detailed mechanical configuration of FUSBOTBS and the associated trajectory and surgical planning have been described elsewhere Citation[23–25], but the procedure is briefly summarized below.
Figure 2. Left: Multiple HIFU transducers mounted on a robotic end-effector submerged in a water tank. Right: The GUI for planning and calibration. [Color version available online.]
![Figure 2. Left: Multiple HIFU transducers mounted on a robotic end-effector submerged in a water tank. Right: The GUI for planning and calibration. [Color version available online.]](/cms/asset/3d3c8dc0-283b-487e-a120-ae5ab31158a1/icsu_a_358850_f0002_b.gif)
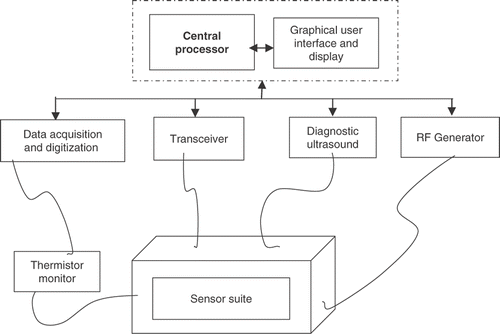
The HIFU transducers are pre-calibrated and mounted on the end-effector in a suitable spatial configuration such that the joint focus lies in a desired target site. Mounted at the central shaft of the end-effector is an imaging ultrasound probe for image guidance and lesion localization during surgery. The robotic end-effector operates in a degassed water tank, to which the target tissue is coupled through a surgical window in the top cover. The test set-up is shown in . The HIFU protocol is remotely controlled using a programmable multi-channel RF amplifier through the RS232 interface. A transceiver unit and other sensors are synchronized through the software using a parallel port. The data acquisition circuit for sensor data is connected to the DAS unit, and its operation is controlled by the software during HIFU exposure. For each treatment point, the surgeon can specify the power and exposure time for HIFU using a user-friendly graphical interface.
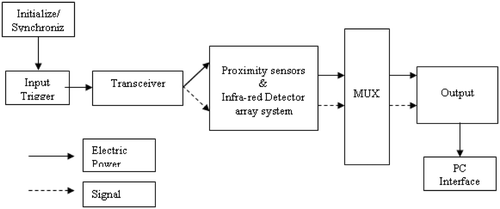
For remote thermometry measurements, various types of sensors were proposed and integrated in the end-effector module of the robotic system. The ultrasound imaging sensors are integrated at both the shaft and slot ring confocal to the HIFU probes. The infra-red sensors (transceivers) and proximity sensors are located at various locations near the end-effector in the robotic workspace. A bead thermocouple was embedded in the test specimen, with provision for variable heights (through holes in the base plate at specified locations for initial calibration purposes). Together, these elements comprised a sensor suite for which the data was acquired, processed and fused at specified intervals during the ablation procedure in order to infer lesion location and thermal feedback.
Diagnostic images from the ultrasound unit are continuously fed to an image grabber inside the PC-based controller. The software contains various components that interact with external devices, such as an RF generator, transceiver, ADC/DSP card and custom-built thermistor reader card for data acquisition and processing (). shows the schematics of the interface for the sensor array with the DAC unit controlled through the software. The control software for system operation is written using Visual C++ and open GL. It has three basic modules: image control, trajectory control and dosage control.
Algorithms for monitoring the lesioning process
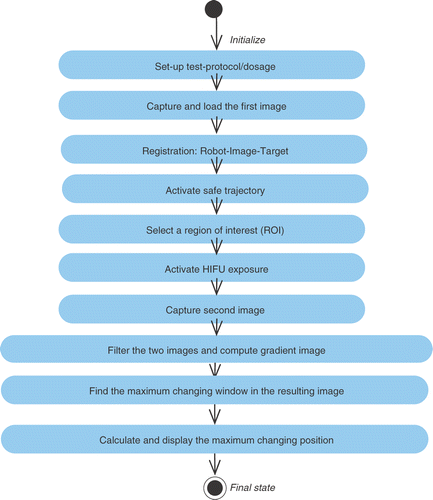
For image guided surgery, the target region, as well as the surgical protocols, are pre-defined by the surgeon and marked with a light pen and/or mouse cursor on a 2D ultrasound image on a touch screen which is continuously captured and displayed on the monitor by the image control module of the control software Citation[23–25]. This step is followed by coordinate transformation between the image-map and operative domain to guide the surgical tools through specified trajectories to the target using the trajectory control module of the control software, which moves the robotic end-effector precisely towards the target defined by the surgeon. This is followed by HIFU exposure, as controlled by the dosage control module. However, the remote lesion is not seen directly on imaging ultrasound and such an open-loop system would be unable to address critical questions such as 1) Has the lesion been created?; 2) Is the position of the lesion the same as that specified pre-operatively?; and 3) Is the local temperature rise in the target area sufficient to create the desired necrosis?
To address some of these important issues, we attempted to incorporate online, non-invasive feedback in the system by introducing two sets of control algorithms for localizing the lesion and estimating the temperature at the localized site, respectively. These were derived and tuned empirically, and integrated with the control architecture of the FUSBOTBS system to close the loop for surgical control (as shown in ). The first closed loop (in the upper part of ) involves image/signal processing to track and calculate the lesion position. Due to the local temperature increase in the targeted focal region, it can be distinguished by its different gray levels in the image compared to those of live tissues if captured immediately after exposure. This difference can be used to locate the created lesion. The image control module displays a cross-section of the sample tissue on the monitor screen, and the position of the specified treatment point (lesion) is mapped to the robot coordinates (using updated data from proprioceptive sensors). Target images are captured before and after HIFU exposure and are processed for gray-level gradients, which are mapped to the pre-calibrated temperature rise. The position feedback is then used to check and update dynamically the robot trajectory for the subsequent treatment points.
Figure 5. Dynamic compensation using lesion position and temperature feedback. [Color version available online.]
![Figure 5. Dynamic compensation using lesion position and temperature feedback. [Color version available online.]](/cms/asset/3775b3b9-8b64-4242-8e2d-a2d9016eecf6/icsu_a_358850_f0005_b.gif)
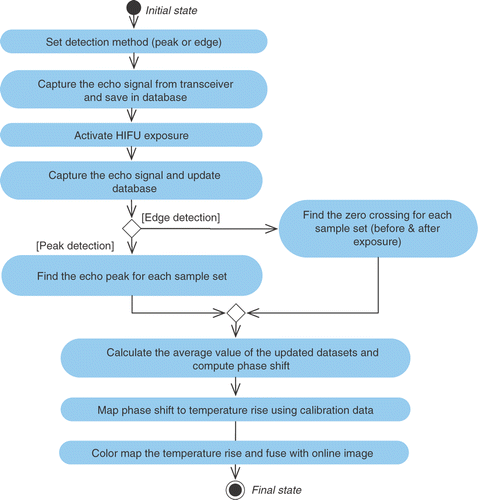
The second closed loop (in the lower part of ) calculates and plots a temperature map in the user-selected region of interest (ROI) using phase shift. One of the existing HIFU probes (or an optional ultrasound probe) is used in transmit-receive mode in the dead time between two consecutive exposures. The changes in the physical properties of the exposed tissue and the acoustic properties such as sound velocity and attenuation coefficient result in a discernable change in echo characteristics. A shift in phase of the ultrasound wave is observed, which is captured using the ultrasound probe and sent to the control software. The user sets the detection mode (to either peak detection or edge detection) which is used to analyze the echo signal. The change in echo shift is used to map the local temperature rise in the lesion area by interpolating a pre-calibrated shift in the temperature rise data set. The temperature information is overlaid on the image and is displayed on the screen as a color map (, and ).
Figure 9. Color map of the tissue region with respect to the selected noise tolerance. [Color version available online.]
![Figure 9. Color map of the tissue region with respect to the selected noise tolerance. [Color version available online.]](/cms/asset/333c198d-705d-417a-9d82-ac30423af5c4/icsu_a_358850_f0009_b.gif)
The arrows in represent logical inter-actions among major sub-sections, while the dashed lines indicate that the HIFU transducers, imaging probe and thermometric probe are all mounted on the robotic end-effector and are hence registered in a common and known coordinate frame. The control software compares the specified position of the treatment point and the calculated lesion position. For four consecutive treatment points (in three adjacent treatment planes), the difference between these positions is averaged and used to adjust the robot movement for subsequent treatment points. The aforementioned participating control algorithms are depicted in the UML activity diagrams in and .
Results and discussion
In this study, the dependence of the ultrasound parameters on temperature and their use for FUS monitoring was investigated. In the first stage of the experiments, a set of thermistors was inserted into the tissue phantom (freshly acquired porcine and lamb adipose tissue or porcine kidneys) at the predicted focus of the HIFU transducers (offset by 1 mm). The representative empirical results presented in this section used a triple set of 2-MHz HIFU probes (Imasonic S.A., Voray, France) focused at 64 mm in both planner and spatial configurations. B-scan image and sensor-suite readings were recorded after exposure. Twenty sets of data were collected using different tissue samples, which were then used to map the echo-change to the temperature at the HIFU-induced lesion. In the second stage of the experiments, the thermistors were removed. Images were captured without the thermistor so that the size and shape of the lesion could be shown more clearly in the B-scan image. An appropriate noise tolerance (based on several empirical results) was added in the lesion-tracking algorithm to avoid the intensity changes (noise) caused by bubbles and misalignment after HIFU ablation.
Offline calibration
Initially, offline thermal monitoring was conducted using calibrated test jigs (precision X–Y tables Citation[20]) and by saving and recalling image data sets from a designated database in order to optimize the offline parameters and interface circuits prior to conducting online experimental studies. The test protocol used in these experiments comprised three probes at 24 W (each), 5 seconds of exposure time, 10 seconds of dead time, and a single sonication at each spot. Tabulated results () show the echo amplitude and delay/shift (in the color map) with respect to the noise tolerance selected during image processing. Tolerance levels 5 and 6 were invariably selected because more than 90% of the noise can be removed: higher levels may distort the lesion, as was observed in several images.
As expected, the lesions were found to be dispersed at lower temperatures, but more localized as the input power (and hence the temperature) was increased. The results were highly promising, with 57 out of 60 lesions being localized correctly using the lesion-searching algorithm. The other three lesions were dispersed/distorted in the macroscopic examination while slicing, preventing the results from being interpreted correctly. shows the image data fused with the temperature map, and and show the pulse amplitude and phase-shift variation with temperature respectively. As can be seen, the pulse amplitude drops suddenly after ∼60°C (inconsistent fluctuations), and readings beyond this were not reliable. However, phase-shift recordings were found to be consistently linear with temperature.
Robotic FUS integrated surgical feedback
The calibrated sensors and probe set were used for empirical tests on various biological phantoms such as porcine/lamb adipose tissue (analogous to human breast tissue) and porcine kidneys. These experiments were repeated with image guided online planning and automated control of trajectory, HIFU dosage parameters, image maps and real-time feedback using the FUSBOT system in surgeon-defined protocols. A test set-up, showing the end-effector and sensory suite with the excised tissue (porcine kidney) through the tank interface of the robotic system is shown in . Some representative results are also shown in for a defined test protocol. The results thus obtained are helpful for understanding lesion adjacency and consistency and track formation in the intervening path, along with online surgical feedback.
Figure 12. (a) The FUSBOT system as seen under the operating table, with target tissue descended through the breast operating window. (b) Lesions induced in porcine kidney with 22 W/8 seconds exposures at 2 mm (top) and 1 mm (bottom) separation. [Color version available online.]
![Figure 12. (a) The FUSBOT system as seen under the operating table, with target tissue descended through the breast operating window. (b) Lesions induced in porcine kidney with 22 W/8 seconds exposures at 2 mm (top) and 1 mm (bottom) separation. [Color version available online.]](/cms/asset/fd3adc9c-619c-4061-aa9e-a7b29f528b56/icsu_a_358850_f0012_b.gif)
Localization and targeting performance of the robotic system
The dependency of desired tissue damage (due to temperature elevation) on parameters governing the thermal dose, was studied during in vitro and ex vivo trials. The role of the robotic manipulator is to position the end-effector (and thus the target lesion) in a pre-defined ROI localized remotely inside the body/target tissue, as well as to mechanically sweep the end-effector in a pre-defined trajectory so as to ablate a given arbitrary shape in three dimensions (to match the dimensions of the tumor/cancer region). In manual manipulations, it is not always possible to control lesion adjacency, dosage distribution and/or coverage of an arbitrary target shape. Various possible lesion patterns have been created and studied at different tissue depths, intervening layers, tissue types and perfusion effects (see results presented in references Citation[20], Citation[21], Citation[23–25]). The system trials using live organs and tissues were conducted under the guidance of our clinical collaborators at the Klinikum Mannheim, as well as resident medical staff in the group. The flexibility of the system in creating various scanning patterns was also demonstrated in these trials; this is not possible with conventional HIFU systems. The results obtained using our surgical system demonstrated very high consistency in terms of lesion dimensions (controlled dosage parameters), precision and repeatability in localization with an image guided surgical protocol and trajectory of approach.
Integrated control: online feedback and trajectory control
As explained earlier, this sub-system was devised for lesion position tracking and thermal monitoring during ablation. Both of these crucial parameters were correlated and fused with the real-time ultrasound imaging information. The sensory sub-system was integrated with robotic proprioceptive sensors for proximity and reach with a diagnostic ultrasound unit through a central processor. Robot and HIFU dosage controls were de-linked for safety reasons. The online images from the diagnostic ultrasound unit were continuously updated on the GUI. Data from various sensors (positioning, imaging and thermometry) were simultaneously acquired during the ablative procedure. The acquired data was synchronized and digitized prior to sending to the central processor.
Several signal attributes involved in dosage computation and the resulting lesion position and extent effect a change in the image and sensory data. Online diagnostic images were processed (the methodology and algorithms were described previously in the Methodology section) and a scanning window was run in the ROI selected by the user in the online image (instead of processing the whole image). An average of the four nearest neighbors was computed to track lesion position correlated with both the target and the end-effector position. Tabulated results for these parameters obtained in various ex vivo tests are presented in . A representative snapshot of the graphical interface, showing an online ultrasound image fused with the lesion position and temperature map is shown in .
Figure 13. Image display on GUI window shown as a result of fusion of sensory data for lesion tracking and thermal mapping. [Color version available online.]
![Figure 13. Image display on GUI window shown as a result of fusion of sensory data for lesion tracking and thermal mapping. [Color version available online.]](/cms/asset/04e2ee00-b00f-48e0-9bca-6ff38a160bef/icsu_a_358850_f0013_b.gif)
Table I. Representative results averaged from 80 experiments in in vitro and ex vivo tissues (porcine adipose and kidneys) for lesion positioning algorithm performance.
Due to the instant temperature increase soon after exposure, the variation in data in terms of the gray-level of the real-time image for lesion localization was registered in various tissue samples. The ultrasound velocity and echo shift were mapped and used to deduce the temperature of the resulting lesions. Tests were conducted in excised porcine and lamb samples using several exposure protocols (, ). Changing exposure parameters and tissue samples resulted in inconsistent change in echo amplitude when measured alone. However, normalized phase-shift and gray-level changes showed consistent outcomes in a controlled environment (a degassed, temperature-controlled bath). Based on our experimental results, the relation between phase shift and local temperature rise is approximately linear below 50°C, after which it changes irregularly. Thus, an estimate of lesioning temperature (>60°C) could be in error: It can only provide a precursor of the range of temperatures reached in the target volumes. A comparison was made between lesions detected by the algorithms and those detected by macroscopic examination after resection, and a close correlation was found.
Conclusions
A technique of fusing online images and data from a dedicated sensor suite, as integrated and implemented in a customized surgical robotic system, FUSBOTBS, for lesion tracking and thermal mapping to provide surgical feedback in FUS applications has been described. Test protocols were conducted in over 80 samples with changing exposure parameters and test settings. Tissue reflective and refractive characteristics are affected during ablation, and this variation in data was mapped and used to deduce the position and temperature of the resulting lesions. Our test results establish the feasibility of this technique under varying dosage protocols. These tests are crucial for judging the performance of the systems in order to deduce HIFU exposure conditions and optimize surgical protocols; select desired multi-probe configurations; and assess the localization and targeting performance of the robotic system.
Lesion positioning and thermal feedback algorithms have been developed. Dynamic lesioning computation helps in automated re-registration of target points for further exposures during treatment when a robotic system is employed. Our FUSBOT systems are based on hybrid supervisory control architecture which allows for crude and fine positioning. We believe that these algorithms can further improve the clinical efficiency of HIFU-based non-invasive cancer treatment. The lesion tracking algorithm has a success rate of up to 100% in detecting lesions for cases where lesions were found by macroscopic examination. Temperature tracking using phase shift is reliable with limited resolution and temperature range; for optimum weighting factors, statistically large samples would be required for tuning. However, in extending this for reliable clinical usage, the following factors would require further attention:
The phase-shift processing algorithm depends on the existence of a reflector, which may be taken as a fixed anatomical feature in close proximity to the ROI and visible in the ultrasound image. Besides the temperature change, phase shift and intensity gradients may also be affected by many other factors, such as the temperature of the water bath and of the tissue under investigation, the formation of bubbles near the ROI, etc. The surgical process and environment must be carefully studied to differentiate the influence of each factor.
The image guided surgery and feedback algorithms require precise alignment of the HIFU transducers, imaging probe, thermometric probe and reflector. Any misalignment could result in undesirable targeting and feedback if manual jigs and fixtures are used. The robotic end-effector is less prone to such misalignment errors with its robust and reliable control logic. Dynamic re-registration would be quite impossible to implement in a manual or automated system. The design of the surgical trajectory and protocol is another important issue that must be addressed.
Feasibility tests in tissue in vitro showed the high potential of these parametric computations for providing feedback during ablation. A statistically large number of in vivo tests in various tissue types would be desirable to ensure clinically reliable outcomes.
Acknowledgments
The authors would like to acknowledge funding support from the Agency for Science, Technology and Research (A*STAR), Singapore, and the Ministry of Education, Singapore. They are also grateful to Storz Medical AG, Switzerland, for clinical sundries and equipment support during the clinical tests.
References
- Chapelon JY, Ribault M, Vernier F, Souchon R, Gelet A. Treatment of localised prostate cancer with transrectal high intensity focused ultrasound. Eur J Ultrasound 1999; 9: 31–38
- ter Haar G, Sinnett D, Rivens I. High intensity focused ultrasound — a surgical technique for the treatment of discrete liver tumors. Phys Med Biol 1989; 34: 1743–1750
- Madersbacher S, Schatl G, Djavan B, Stulnig T, Marberger M. Long-term outcome of transrectal high-intensity focused ultrasound therapy for benign prostatic hyperplasia. Euro Urol 2000; 37: 687–694
- Köhrmann KU, Michel MS, Gaa J, Marlinghaus E, Alken P. High intensity focused ultrasound as noninvasive therapy for multilocal renal cell carcinoma: Case study and review of the literature. J Urol 2002; 167: 2397–2403
- Hynynen K, Pomeroy O, Smith DN, Huber PE, McDannold NJ, Kettenbach J, Baum J, Singer S, Jolesz FA. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: A feasibility study. Radiology 2001; 219: 176–185
- Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 2001; 220: 640–646
- Gianfelice D, Khiat A, Amara M, Belblidia A, Boulanger Y. MR Imaging-guided focused US ablation of breast cancer: Histopathologic assessment of effectiveness–initial experience. Radiology 2003; 277: 849–855
- Stafford RJ, Price RE, Diederich CJ, Kangasniemi M, Olsson LE, Hazle JD. Interleaved echo-planar imaging for fast multiplanar magnetic resonance temperature imaging of ultrasound thermal ablation therapy. JMRI 2004; 20(4)704–714
- Souchon R, Rouviere O, Gelet A, Detti V, Srinivasan S, Ophir J, Chapelon JY. Visualisation of HIFU lesions using elastography of the human prostate in vivo: Preliminary results. Ultrasound Med Biol 2003; 29(7)1007–1015
- Chauhan S, Singh VR, Chakarvarti SK. Non-invasive measurement of temperature in biological media under ultrasonic irradiation. J Acoust Soc India 1990; 18(3–4)273–278
- Singh VR, Chauhan S, Yadav S, Chakarvarti SK. Ultrasonic velocity as a measure of temperature non-invasively in biological media. Appl Acoustics 1989; 29: 73–80
- Maass-Moreno R, Damianou CA, Sanghvi NT. Noninvasive temperature estimation in tissue via ultrasound echo-shifts. Part II. In vitro study. J Acoust Soc Am 1996; 100(4)2522–2530
- Seip A, Ebbini E. Non-invasive estimation of tissue temperature response to heating fields using diagnostic ultrasound. IEEE Trans Biomed Eng 1995; 42(8)828–839
- Bailey MR, Vaezy S, Yuen JC, Anand A, Miller NA, Kaczkowski PJ, Crum LA. Bubbles and acoustic image-guided high intensity focused ultrasound. J Acoust Soc Am 2001; 110(5)2643
- Makin IRS, Mast TD, Barthe PG, Slayton MH. B-scan imaging and thermal lesion monitoring using miniaturized dual-functionality ultrasound arrays. Proceedings of the 2004 IEEE Ultrasonics Symposium. 23–27 August, 2004; 3: 1788–1791
- Chen SS, Humphrey JD. Heat-induced changes in the mechanics of a collagenous tissue: Pseudoelastic behavior at 37°C. J Biomech 1998; 31: 211–216
- Chauhan S, Lowe MJS, Davies BL. A multiple focused probe approach for high intensity focused ultrasound based surgery. Ultrasonics 2001; 39(1)33–44
- Davies BL, Chauhan S, Lowe MJ. A robotic approach to HIFU based neurosurgery. Proceedings of the First International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI '98), WM Wells, A Colchester, S Delp. Cambridge, MA, October 1998. Lecture Notes in Computer Science 1496., Springer, Berlin 1998; 386–396
- Chauhan S. Field modelling for multiple focused ultrasound transducers. Proceedings of the 8th IEEE International Conference on Mechatronics and Machine Vision in Practice (M2VIP 2001). Hong Kong August, 2001; 27–29
- Haecker A, Michel MS, Alken P, Köhrmann KU, Chauhan S. Multiple focused probes for high intensity focused ultrasound: an experimental investigation. Proceedings of the 20th World Congress on Endourology and Shockwave Lithotripsy. GenoaItaly September, 2002; 19–22
- Amir H, Dash AK, Chauhan S. A test harness for ultrasonic instrumentation using mechatronic means. Proceedings of the 11th IEEE International Conference on Mechatronics and Machine Vision in Practice (M2VIP 2004). Macao December, 2004; 203–208
- Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol 1948; 1: 93–122
- Chauhan S. A mechatronic system for non invasive treatment of the breast cancers. Proceedings of the 9th IEEE International Conference on Mechatronics and Machine Vision in Practice (M2VIP 2002). Chiang MaiThailand September, 2002; 359–365
- Häcker A, Michel MS, Knoll T, Marlinghaus E, Alken P, Köhrmann KU, Chauhan S. Multiple versus single ultrasound probes for tissue ablation by high intensity focused ultrasound: An experimental investigation. J Urol 2003; 169(Suppl 4)
- Chauhan S, Li JR, Mishra R, Lim WK, Hacker A, Michel MS, Alken P, Köhrmann KU. Experiences using a special purpose robot for focal ultrasound based tissue ablation. Proceedings of the International Symposium on Therapeutic Ultrasound (ISTU 04). KyotoJapan September, 2004; 18–20
![Figure 8. Image data fusion correlated with temperature map. [Color version available online.]](/cms/asset/a31672d9-972e-4855-8111-9826c23d1e71/icsu_a_358850_f0008_b.gif)
![Figure 10. Variation of acoustic pulse amplitude with temperature rise (base temperature of the bath = 25°C). [Color version available online.]](/cms/asset/a598bad8-5891-4091-8a26-56116ce374fb/icsu_a_358850_f0010_b.gif)
![Figure 11. Phase shift versus temperature. [Color version available online.]](/cms/asset/96e3dc40-6769-4ef0-b427-895cbd242724/icsu_a_358850_f0011_b.gif)
![Figure 14. Gray level versus power for various exposure durations in excised porcine (a) and lamb (b) tissues. [Color version available online.]](/cms/asset/b3ac4fca-6eb0-449a-a300-55f75ea8a136/icsu_a_358850_f0014_b.gif)