Abstract
Introduction: Previous studies have shown that total disc replacement (TDR) resulted in significantly better restoration of disc-space height and significantly less subsidence than anterior interbody fusion with BAK cages. Clinical outcomes and flexion/extension range of motion correlated with the accuracy of surgical placement of the CHARITÉ™ artificial disc. False positioning of the artificial disc leads to spondylarthrosis and disc degeneration of the adjacent segment, and exclusive use of a C-arm could cause such false positioning (due to the parallax effect). The objective of this study was to test and evaluate the accuracy of navigated artificial disc replacement as performed by a spine surgeon without a prior learning curve. In each case, the placement position achieved by the surgeon was compared with the preoperatively planned position for that specimen.
Materials and Methods: Lumbar intervertebral disc prostheses (CHARITÉ™, DePuy Spine) were placed using an image guidance technique (BrainLAB VectorVision system) in ten human cadaveric spine specimens. A total of 15 such disc replacements were performed using navigation. Post-instrumentation accuracy was assessed by a computer on the basis of CT scans.
Results: The placement of the disc was assessed as ideal (<3 mm from the planned position), suboptimal (3–5 mm from the planned position) or poor (>5 mm from the planned position). Only three disc prostheses were placed suboptimally, and none was poorly placed. Placement in the coronal plane was significantly better than in the other planes.
Discussion: Navigation is a useful instrument in the hands of the spine surgeon, enabling an ideal placement of the disc prosthesis. Navigation offers greater accuracy and less inter-procedural variation than standard fluoroscopy (due to the parallax effect). As accurate (ideal or suboptimal) placement correlates with good clinical outcome, further clinical studies on the navigation of TDR are essential. In this present study, the disc replacement was performed by a surgeon without experience in total disc replacement, indicating that prior completion of a learning curve was not essential.
Introduction
Interbody fusion is considered to be the gold standard for open surgical treatment of symptomatic discogenic back pain. The goal is the elimination of motion at the chosen segments Citation[1]. An alternative treatment option, designed to preserve or improve physiologic motion while minimizing junctional stresses and protecting the adjacent levels against nonphysiologic loading Citation[2–4], is total disc arthroplasty, which has been actively practiced in Europe over the last decade with varying degrees of success Citation[2]. Despite the early clinical success of total disc replacement (TDR) Citation[5–8], the incidence of complications has been reported to be anywhere from 2.9% to 12% Citation[5],Citation[9]. Long-term studies of one-level lumbar arthroplasty report unsatisfactory results in 18% of cases. An incorrect surgical indication, malpositioning and incorrectly sized implants appear to be the main causes of unsatisfactory outcomes in TDR Citation[10].
The CHARITÉ™ artificial disc (DePuy Spine, Inc., Raynham, MA; ) was designed to duplicate the kinematics and dynamics of a normal motion segment in the lumbar spine while restoring intervertebral space height and motion segment flexibility Citation[11],Citation[12]. It is comprised of two cobalt-chromium alloy endplates and a sliding ultra-high-molecular-weight polyethylene (UHMWPE) core. The mobile, sliding core design allows the core to shift dynamically during normal spinal motion, moving posteriorly in flexion and anteriorly in lumbar extension.
Gertzbein et al. and White and Panjabi Citation[13],Citation[14] described the physiologic pattern of motion in the normal functioning intervertebral disc as an ellipse rather than a single point. An optimally placed artificial prosthesis is centered in the anterior-posterior (AP) view in neutral rotational alignment with regard to the midline. In the sagittal plane the center of the artificial disc should be positioned 2 mm dorsal of the vertebral body midline Citation[3],Citation[15]. An implant that is placed within 3 mm of the placement described above is considered “ideal” Citation[9]. Incorrectly sized implants and poor positioning within the disc space may be a significant source of unsatisfactory results in TDR. Malpositioning potentially predisposes the segment to asymmetric loading, risking premature implant wear, implant loosening, and nonphysiologic stresses on adjacent segments. Most disc prostheses are inserted and analyzed using fluoroscopy Citation[16].
A possible solution to the problem of disc implantation accuracy may be the use of hands-free surgical navigation systems. Surgical navigation systems–or, more precisely, surgical freehand navigation systems–are characterized by the fact that the hand of the physician guides conventional instruments whose positions can be assessed in 3D space Citation[17]. A similar navigation system was developed to achieve better positioning of the disc replacement - in this case the CHARITÉ™ prosthesis - and avoid collateral damage due to malpositioning. Possible false positioning of the artificial disc could be avoided by not relying exclusively on a C-arm due to the parallax effect Citation[18].
The goal was to assess the accuracy of placement of total disc prostheses using a navigation system (BrainLAB), with the actual placement of the disc being compared to a pre-planned “ideal” placement. The planning of the ideal placement was performed by a surgeon (M.R.) with experience of total disc replacement. Another goal was to evaluate whether a learning curve was essential by having an experienced spine surgeon perform total disc replacement without having completed such a learning curve beforehand.
The ultimate goal of this work is the integration of these operative steps into the surgical technique of disc prosthesis placement.
Materials and methods
Cadavers
Ten cadavers (6 female, 4 male) were purchased for this study from Toro University (Las Vegas, NV). The average age of the cadavers was 71.8 years (range: 36–88 years), and the average specimen body mass index was 21.61 (range 16.0–24.86). The implants (CHARITÉ™ prostheses of size 3) and the necessary instruments were provided by DePuy Spine, Inc. (Raynham, MA). The study was conducted in laboratory facilities at Touro University.
Preoperative imaging and data analysis
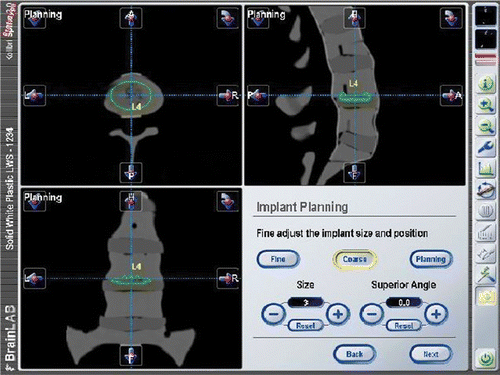
Prior to surgery, CT scans were obtained of the lumbar spine of each specimen to exclude the presence of any structural lesions or pronounced spinal or pelvic deformity at the levels where surgery was intended. An additional exclusion criterion for implant placement was a history of previous spine or pelvis surgery. In cases where anatomical change (e.g., osteoporotic subsidence, deformation of the vertebra, or synostosis of the facet joints) was detected, the affected level was not taken into consideration for the statistical analysis. After thawing, the cadavers were placed in a supine position with a posteriorly applied radiolucent bump to aid in lordosing the lower lumbar spine. The CTs were loaded into the navigation software, and an experienced spine surgeon (M.R.) identified an “ideal” placement of the prosthesis planned at each lumbar level ().
Surgical technique
The surgical technique for implanting an artificial disc is in many ways similar to performing an anterior lumbar interbody fusion (). The size of the implant is determined with the aid of the preoperative CT scan.
Figure 3. Incision for the ventral trans- or extraperitoneal surgical approach for the disc implantation.
After performing a complete discectomy, vertebra endplates were identified and preserved in order to provide a firm base for mechanical stability and reduce the potential for subsidence.
Preparation for navigation
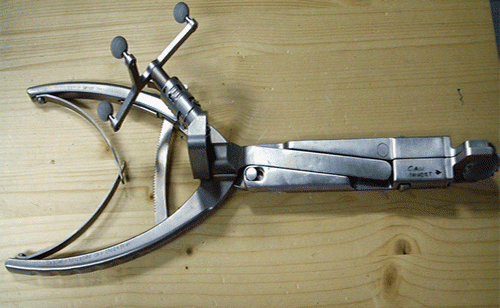
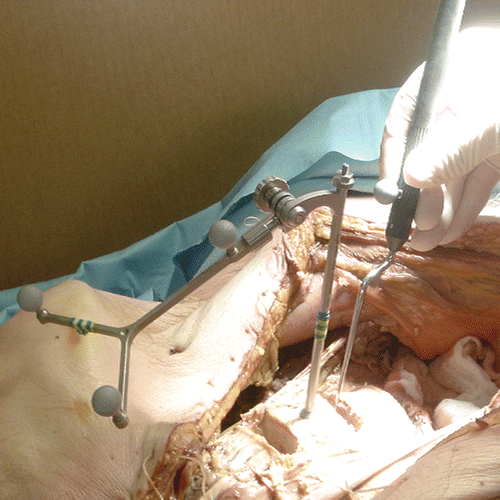
The navigation system technology used in this study (VectorVision system, BrainLAB, Feldkirchen, Germany) works via vector analysis of reflective reference arrays that are attached to standard surgical instrumentation as well as to a reference array that was temporarily anchored in the vertebral body above the operative disc space. An optical tracking system (Polaris, Northern Digital Inc., Waterloo, Ontario, Canada) in the operating room tracked the reflective arrays located on the instrumentation and was able to determine the orientation and location of instrumentation in relation to a fixed reference on the patient's vertebral body. The attachment of reflective arrays to the non-surgical end of the standard CHARITÉ insertion forceps was necessary to properly track the instrumentation through space. Three reflective markers were attached to the insertion forceps, the same number as were present on the reference array ( and ). The navigation software was used to track the CHARITÉ implant not as an independent unit, but as the distal end of the insertion forceps. As a result, the navigation software did not require any modifications to the CHARITÉ implant itself. In addition to the insertion instrument, markers were also placed on the reference array and the pointer. The latter is used to point at visible anatomy to verify the accuracy of the navigation system's display (). Analysis of the reflected signals shows which markers belong to the same instrument and identifies the instrument.
Reference array
The insertion of the reference array in the above vertebra integrates a coordinate system used as a template for the CT scan and its relation to the patient's anatomy. The stability of the inserted reference array during the navigation phase of the operation was of great importance. The reference array was placed at a 30–45° angle to the medial line of the cranial vertebral body, and the full length of the thread was inserted into the vertebra to provide the necessary stability.
The reference marker array was oriented towards the cameras of the tracking system and adjusted to ensure visibility to the cameras throughout the surgical procedure (see ).
Intraoperative imaging and registration
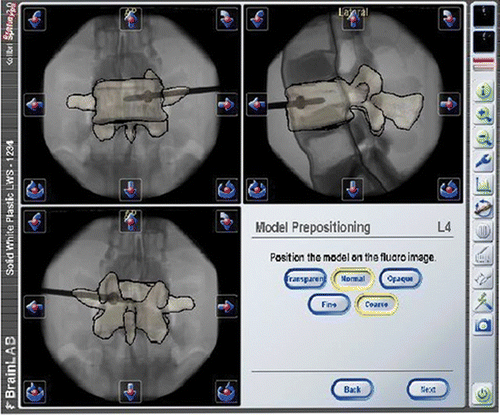
To perform a fluoroscopy-to-CT registration, an AP and lateral fluoroscopic image were acquired with a standard C-arm. The accuracy of registration was verified using the pointer to indicate anatomical landmarks.
After prepositioning of the segmented vertebra on the AP and lateral fluoroscopic images, the matching was controlled using the overlaid contours ().
Data analysis
After implantation, postoperative CTs were acquired for all cadaver specimens. The postoperative CTs were loaded into the navigation software and the location of the implanted prostheses was determined by manual labeling of the four spikes closest to the corners of the cranial endplate (). Using the navigation software, the postoperative location of the implant was compared to the location of the “ideal” prosthesis, which was planned prior to the surgery. Using a prototype software (i-Plan 2.0, BrainLAB), the comparison between the planned and postoperative positions was accomplished by marking the outer spikes of the CHARITÉ prosthesis. The i-Plan 2.0 software is a developmental prototype not connected to a specific BrainLAB product. The descriptive statistics used include the median, standard deviation, minimum and maximum, and the 95% confidence interval for the mean. The box plots display the 25th and 75th percentiles (interquartile range) of the data set, the median and the mean.
The radiographic placement was categorized as described by McAfee et al. Citation[9]. Group I, or “ideal placement”, was defined as placement of the artificial disc within 3 mm of exact central placement (in right/left deviation, cranial/caudal deviation and anterior/posterior deviation). Group II, or “suboptimal placement”, was defined as placement deviation greater than 3 mm but less than 5 mm from exact central placement in each plane. Group III, or “poor placement”, was defined as placement deviation of greater than 5 mm from exact central placement in each plane.
Results
Two levels were not taken into consideration for statistical analysis due to the above-mentioned exclusion criteria. shows the deviation in each plane.
Table I. Deviation from the preoperatively planned ideal position of the artificial disc. Numbers in bold indicate suboptimally placed prostheses.
For the coronal plane (= AP radiograph) the deviation from the planned position was 0.858 ± 0.62 mm. The maximum value was 2.321 mm and the minimum value was 0.139 mm. Twenty-five percent of the navigated CHARITÉ discs deviated by no more than 0.4097 mm, and 75% were less than 1.223 mm from the planned position (). The 95% confidence interval of mean was 0.375 mm. All artificial discs were ideally placed (with less than 3 mm of deviation).
Figure 7. Placement in the coronal plane is significantly better than in the sagittal or craniocaudal planes.
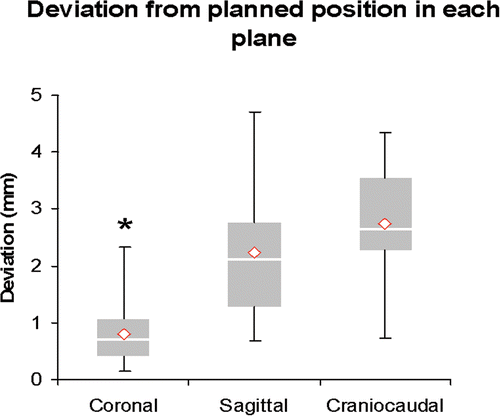
For the midsagittal plane (= lateral radiograph) the deviation from the planned position was 2.235 ± 1.327 mm. The maximum value was 4.712 mm and the minimum value was 0.687 mm. Twenty-five percent of the navigated CHARITÉ discs deviated by no more than 1.284 mm, and 75% were less than 2.775 mm from the planned position (). The 95% confidence interval of mean was 0.772 mm. Two artificial discs were suboptimally placed (Group II).
In the craniocaudal direction, the maximum value for the deviation from the planned position was 4.353 mm, and the minimum value was 0.725 mm. The maximum value was 4.533 mm and the minimum value was 0.753 mm. Twenty-five percent of the navigated CHARITÉ discs deviated by no more than 2.2877mm and 75% were less than 3.54 mm from the planned position (). The 95% confidence interval of mean was 0.602 mm. Four artificial discs were suboptimally placed (Group II), but none of the discs had a poor placement.
Only two discs in the midsagittal plane and four in the craniocaudal plane had a suboptimal placement. None of the navigated disc prostheses showed a poor placement. Disc placement in the coronal plane was significantly better (p < 0.05) than in the other planes ().
Discussion
Despite the early clinical success of total disc replacement (TDR) Citation[5–8], the incidence of complications has been reported to be anywhere from 2.9% to 12% Citation[5],Citation[9]. Long-term studies of one-level lumbar arthroplasty report unsatisfactory results in 18% of cases. An incorrect surgical indication, malpositioning and incorrectly sized implants appear to be the main causes of unsatisfactory results in TDR [0].
Surgical navigation technology is designed to improve surgical accuracy with regard to implant placement Citation[19]. The introduction of CT-image-based computer guidance systems into spine surgery allows the surgeon for the first time to navigate an instrument inside the vertebra with the help of real-time 3D images. Several clinical studies have shown that this technique improves the accuracy and safety of pedicle screw insertion Citation[20–26]. This improvement has been demonstrated with regard to lumbar and thoracic pedicle screw insertion Citation[27],Citation[28] and sacroiliac screw placement. Similarly improved accuracy has been reported for acetabular cup placement Citation[29–31]. According to Grange et al. Citation[32], optoelectronic navigation systems are likely to be more precise compared to electromagnetic navigators. Furthermore, optoelectronic systems are not affected by ferromagnetic influences in the environment. The data obtained in this study suggest that existing image guidance may have a role in intervertebral disc placement.
Most disc arthroplasties are inserted and analyzed using fluoroscopy Citation[9]. For this approach, a pin is positioned in the estimated midline of each vertebral body. Despite the considerable time spent in gaining collinear fluoroscopic guidance, errors relating to parallax and skewed spinous processes may nevertheless result in an incorrect midline assessment Citation[18], with consequent adverse asymmetries in implant positioning Citation[33].
Using the operative technique with navigation of the disc replacement as presented in this paper, the surgeon avoids the parallax effect and the additional inaccuracy associated with use of the C-arm. Moreover, the surgeon has a continuous visual confirmation of the midline with a CT scan, which facilitates continuous and accurate control of the position of the spinal process and the facet joints relative to the prosthesis.
Ideal placement correlated significantly with better clinical outcomes at 2 years as compared to outcomes with poor placement, with a significant trend toward better clinical outcomes with improved accuracy in device placement. Flexion/extension segmental range of motion correlated significantly with artificial disc placement, with no significant difference being observed between the ideal and suboptimal groups Citation[9],Citation[34]. The zone of ideal placement for the CHARITÉ™ artificial disc is large and forgiving in both planes (±10 mm). In this cadaver study we showed that the navigation of the total disc replacement led to ideal or, in some cases, suboptimal placement. A poor placement was not observed with any of the navigated CHARITÉ™ discs.
This study showed that the placement in the coronal plane (centralization of the prosthesis) was significantly better than in the other planes. This is related to the anatomical limitation in each plane. In the midsagittal plane (= lateral radiograph) the lordosis angle of the level could influence how closely the prosthesis was positioned to the posterior aspect of the vertebral body. In the craniocaudal plane the anatomical limitations are the endplates of the vertebra and the stiffness of the ligaments.
Honl et al. Citation[29],Citation[30] showed that accurate placement of an acetabular cup did not depend on the experience of the surgeon when a navigation system was used. In our study, the operations were performed by an experienced spine surgeon (K.K.) without experience in total disc replacement. Given the fact that no artificial disc was poorly placed, we suggest that navigation of total disc replacement does not demand prior completion of a learning curve. Image guidance systems appear to offer significant advantages over conventional fluoroscopy with regard to implant placement accuracy. Whether such improved positioning translates into improved functional and clinical outcomes will be assessed in a longer-term prospective clinical analysis.
Acknowledgments
The authors with to thank BrainLAB Germany, Feldkirchen, Germany; BrainLAB USA, Westchester, IL; DePuy Spine, Inc., Raynham, MA; and DePuy Spine Germany, Kirkel-Limbach, Germany, for their assistance with this research.
References
- Fritzell P, Hagg O, Wessberg P, Nordwall A. 2001 Volvo Award Winner in Clinical Studies: Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine 2001; 26(23)2521–2532, discussion 2532–2524
- Cunningham B, Dmitriev A, Hu N, McAfee P. General principles of total disc replacement arthroplasty: seventeen cases in a nonhuman primate model. Spine 2003; 28(20)S118–S124
- Büttner-Janz K, Hochshuler S, McAfee P. The Artificial Disk. Springer-Verlag, Berlin 2003
- Link H. History, design and biomechanics of the LINK SB Charité artificial disc. Eur Spine J 2002; 11(Suppl 2)S98–S105
- Cinotti G, David T, Postacchini F. Results of disc prosthesis after a minimum follow-up period of 2 years. Spine 1996; 21(8)995–1000
- Lemaire JP, Skalli W, Lavaste F, Templier A, Mendes F, Diop A, Sauty V, Laloux E. Intervertebral disc prosthesis. Results and prospects for the year 2000. Clin Orthop Relat Res 1997; 337: 64–76
- McAfee PC, Fedder IL, Saiedy S, Shucosky EM. Cunningham BW. SB Charité disc replacement: report of 60 prospective randomized cases in a US center. J Spinal Disord Tech 2003; 16(4)424–433
- Zeegers WS, Bohnen LM, Laaper M, Verhaegen MJ. Artificial disc replacement with the modular type SB Charité III: 2-year results in 50 prospectively studied patients. Eur Spine J 1999; 8(3)210–217
- McAfee PC, Cunningham B, Holsapple G, Adams K, Blumenthal S, Guyer RD, Dmietriev A, Maxwell JH, Regan JJ, Isaza J. A prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITÉ artificial disc versus lumbar fusion. Part II. Evaluation of radiographic outcomes and correlation of surgical technique accuracy with clinical outcomes. Spine 2005; 30(14)1576–1583, discussion E1388–E1590
- David T. Long-term results of one-level lumbar arthroplasty: minimum 10-year follow-up of the CHARITÉ artificial disc in 106 patients. Spine 2007; 32(6)661–666
- Büttner-Janz K. Optimal minimally traumatic approach for the SB Charité Artificial Disc. Eur Spine J 2002; 11(Suppl 2)S111–S114
- Büttner-Janz K, Hahn S, Schikora K, Link H. [Basic principles of successful implantation of the SB Charité model LINK intervertebral disk endoprosthesis]. Orthopade 2002; 31(5)441–453
- Gertzbein S, Seligman J, Holtby R, Chan K, Ogston N, Kapasouri A, Tile M. Centrode characteristics of the lumbar spine as a function of segmental instability. Clin Orthop Relat Res 1986; 208: 48–51
- White A, Panjabi M. Clinical Biomechanics of the Spine. Willams, & Wilkins, Philadelphia, PA: Lippincott 1990
- Smith HE, Vaccaro AR, Yuan PS, Papadopoulos S, Sasso R. The use of computerized image guidance in lumbar disk arthroplasty. J Spinal Disord Tech 2006; 19(1)22–27
- Fujiwara A, Tamai K, An HS, Lim TH, Yoshida H, Kurihashi A, Saotome K. Orientation and osteoarthritis of the lumbar facet joint. Clin Orthop Relat Res 2001; 385: 88–94
- Schlenzka D, Laine T, Lund T. [Computer-assisted spine surgery: principles, technique, results and perspectives]. Orthopade 2000; 29(7)658–669
- Mathews HH, Eisermann L, Le Huec J-C. Total disc arthroplasty using Maverick total disc replacement. Spinal Arthroplasty, a New Era in Spine Care, RD Guyer, JE Zigler. Quality Medical, St. Louis, MO 2005; 224–238
- Austin MS, Vaccaro AR, Brislin B, Nachwalter R, Hilibrand AS, Albert TJ. Image-guided spine surgery: a cadaver study comparing conventional open laminoforaminotomy and two image-guided techniques for pedicle screw placement in posterolateral fusion and nonfusion models. Spine 2002; 27(22)2503–2508
- Geerling J, Gösling T, Gösling A, Ortega G, Kendoff D, Citak M, Krettek C, Hüfner T. Navigated pedicle screw placement: Experimental comparison between CT- and 3D fluoroscopy-based techniques. Comput Aided Surg 2008; 13(3)157–166
- Amiot LP, Bellefleur C, Labelle H. [In vitro evaluation of computer-assisted pedicle screw system]. Ann Chir 1997; 51(8)854–860
- Bolger C, Wigfield C, Melkent T, Smith K. Frameless stereotaxy and anterior cervical surgery. Comput Aided Surg 1999; 4(6)322–327
- Jacob AL MP, Baumann B, Suhm N, Steinbrich W, Regazzoni P (1999) Interactive single-step navigation in iliosacral screw fixation of sacral fractures and fracture dislocations. Proceedings of the Fourth Symposium on Computer Assisted Orthopaedic Surgery (CAOS ’99), DavosSwitzerland, March, 1999, LP Nolte, R Ganz. Hogrefe & Huber, GöttingenGermany, 147–192
- Kalfas IH, Kormos DW, Murphy MA, McKenzie RL, Barnett GH, Bell GR, Steiner CP, Trimble MB, Weisenberger JP. Application of frameless stereotaxy to pedicle screw fixation of the spine. J Neurosurg 1995; 83(4)641–647
- Laine T, Makitalo K, Schlenzka D, Tallroth K, Poussa M, Alho A. Accuracy of pedicle screw insertion: a prospective CT study in 30 low back patients. Eur Spine J 1997; 6(6)402–405
- Merloz P, Tonetti J, Pittet L, Coulomb M, Lavallée S, Sautot P. Pedicle screw placement using image guided techniques. Clin Orthop Relat Res 1998; 354: 39–48
- Mirza SK, Wiggins GC, Kuntz C, IV, York JE, Bellabarba C, Knonodi MA, Chapman JR, Shaffrey CI. Accuracy of thoracic vertebral body screw placement using standard fluoroscopy, fluoroscopic image guidance, and computed tomographic image guidance: a cadaver study. Spine 2003; 28(4)402–413
- Kosmopoulos V, Schizas C. Pedicle screw placement accuracy: a meta-analysis. Spine 2007; 32(3)E111–E120
- Honl M, Schwieger K, Gauck CH, Lampe F, Morlock MM, Wimmer MA, Hille E. [Comparison of total hip replacements cup orientation and position. Navigation vs. conventional manual implantation of hip prostheses]. Orthopade 2005; 34(11)1131–1136
- Honl M, Schwieger K, Salineros M, Jacobs J, Morlock M, Wimmer M. Orientation of the acetabular component. A comparison of five navigation systems with conventional surgical technique. J Bone Joint Surg Br 2006; 88(10)1401–1405
- Stiehl JB, Heck DA, Jaramaz B, Amiot LP. Comparison of fluoroscopic and imageless registration in surgical navigation of the acetabular component. Comput Aided Surg 2007; 12(2)116–124
- Grange S, Bunker T, Cooper J, Waldhausen S (1999) Comparing electro-optical and electromagnetic guidance in the preparation in minimal access surgical training enviroments. Proceedings of the Fourth Symposium on Computer Assisted Orthopaedic Surgery (CAOS ’99), DavosSwitzerland, March, 1999, LP Nolte, R Ganz. Hogrefe & Huber, GöttingenGermany, 36
- Guyer RD, Burd TA, Hochschuler SH. Total disc replacement: implants and clinical results. Spinal Arthroplasty a New Era in Spine Care, RD Guyer, JE Zigler. Quality Medical, St. Louis, MO 2005; 180–222
- McAfee P, Cunningham B, Hayes V, Sidiqi F, Dabbah M, Sefter J, Hu N, Beatson H. Biomechanical analysis of rotational motions after disc arthroplasty: implications for patients with adult deformities. Spine 2006; 31(19 Suppl)S152–S160