Abstract
A novel technique of using both a navigation system and an endoscope in intra-lesional curettage of benign bone tumors enables safe and adequate tumor removal via a minimal access approach. We performed curettage of benign bone tumors in five consecutive patients (4 female, 1 male, mean age 31.4 years) using a commercial CT-based navigation system supplemented by visual guidance through a shoulder arthroscope. The bone defect was filled with bone cement in four patients and with artificial bone substitute in one patient. Mean follow-up time was 8.8 months (range: 7–12 months). Mean duration of surgery was 144 min (range: 120–165 min). Mean wound length of each portal site was 19.5 mm (range: 15–25 mm). All patients could achieve a full range of joint movement and walk unaided at 4 weeks post-surgery. No local recurrence was noted.
Introduction
Conventionally, benign bone tumors have been successfully treated with intra-lesional curettage and local adjuvants. A cortical window is usually made over the tumor, which is then curetted and the remaining osseous surface of the cavity cleared using a high-speed burr.
Arthroscopic assisted excision of benign bone tumors has been reported, but only for intra-articular lesions Citation[1–3]. This method requires intraoperative 2D fluoroscopic images for accurate localization of the tumor and positioning of the instruments. Case reports of extra-articular endoscopic assisted resection of benign bone tumors have also been published Citation[4], Citation[5]. In these cases, high-intensity illumination with magnified endoscopic imaging enabled excellent visual inspection of the tumor cavity and a visual assessment of the adequacy of its clearance. In selected patients, this minimal access approach could minimize morbidity and facilitate rapid functional recovery without compromising oncological principles.
The use of a navigation system in musculoskeletal tumor resection has recently been described Citation[6], Citation[7]. The system enables the surgeon to integrate preoperative information concerning local anatomy in relation to the tumor. The instant and real-time rendition of 3D anatomical images provides stark visual feedback, allowing surgeons to obtain a precise orientation with reference to underlying anatomy and the anatomical extent of the tumor. Early results, particularly in the context of the resection of difficult malignant tumors, have been encouraging. Benign bone tumors such as osteoid osteoma can be precisely localized intraoperatively with image guided computer assisted navigation and Iso-C3D fluoroscopy Citation[8]. Such tumors can then be resected using minimally invasive navigated techniques and fine surgical tools that are guided and spatially controlled. However, patients are exposed to additional radiation during the image acquisition required at surgery, and assessment of tumor clearance is not possible without actually seeing the curetted cavity.
A combination of computer navigation and endoscopic techniques has been successfully applied to the resection of basal skull tumors in selected patients Citation[9]. This approach combines the advantages of a less invasive minimal access approach to achieve a precise yet thorough ablation of the tumor. In this paper we report our early experience of using a similar approach, wherein Navigation Endoscopic Assisted Tumor (NEAT) surgery was used to treat five patients with benign bone tumors in the extremities.
Patients and methods
Between July 2008 and December 2008, intra-lesional curettage was performed in five patients with benign bone tumors with the assistance of a computer navigation system and an endoscope. The mean age of the patients was 31.4 years (range: 17–47 years) (). Only tumors without extra-osseous extension were included.
Table I. Details of five cases with navigation endoscopic assisted intra-lesional curettage.
The distal femur was involved in three patients (2 with giant cell tumors, 1 with a chondromyxoid fibroma), the proximal femur in one patient (a chondroblastoma), and the proximal tibia in the last patient (a giant cell tumor). Intraoperative guidance was thought to be necessary because of anticipated difficulties in localizing the exact position of the tumor or in assessing the adequacy of tumor clearance following curettage via a minimal access approach. All tumor removals were performed by the same surgeon (K.C.W. and S.M.K.).
Preoperative planning
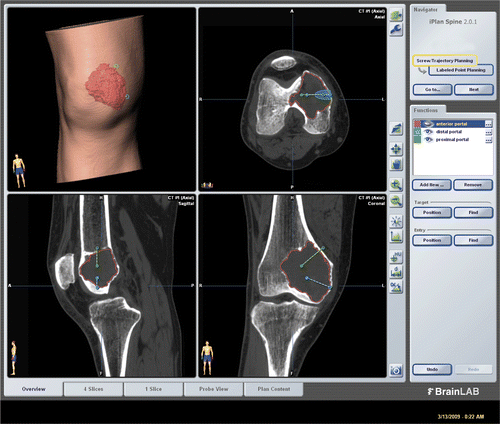
Preoperative CT and MRI examinations of each patient were performed. The preoperative CT was acquired at the same setting as was used when the patient underwent tissue biopsy under CT image guidance at the Radiology Department. Axial CT images of the lesion and surrounding area were acquired using a 16-detector scanner (General Electric LightSpeed, Milwaukee, WI). Slices with 0.625-mm thickness were obtained using a soft tissue algorithm. Radiological data were obtained and imported into the CT-based navigation system (VectorVision, BrainLAB, Feldkirchen, Germany). Because no specific software has been developed for resection of benign bone tumors, we used software developed to assist the placement of pedicle screws in the spine (BrainLAB iPlan Spine, version 2.0.1). The CT and MR images were fused using navigation software and the tumor margin demarcated on the fused images. The navigation software generated a 3D bone and tumor model, which greatly facilitated preoperative planning (). The tumor volume could be calculated and used to estimate the amount of artificial bone graft and bone cement required to fill the curetted tumor cavity. Two portal sites for the introduction of working instruments and the endoscope were defined and marked using virtual screws. The locations of the working portals were planned so that every nook and corner of the tumor cavity could be reached.
Figure 1. CT and MR images for Case 2 were fused in the navigation system and the tumor delineated from the fused image datasets. The tumor volume (outlined in red) can be calculated, enabling an estimate of the amount of bone cement or artificial bone graft required to fill the bone defect after intra-lesional curettage. The locations of working portals were planned and marked by virtual pedicle screws.
Intraoperative navigation and surgical curettage
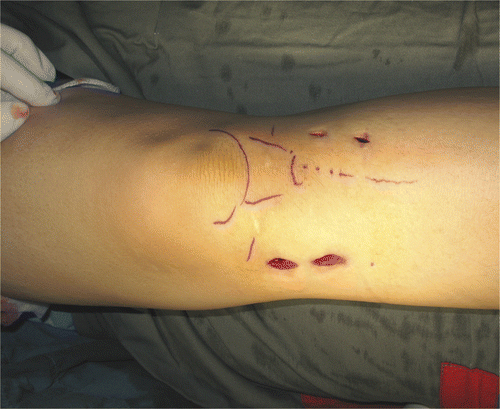
Each intra-lesional curettage was performed using navigation and endoscopic assistance (). A dynamic reference tracker was attached to the bone in which the tumor was located. This enabled us to carry out the registration process and match precisely the operative anatomy to the virtual image generated by the navigation software. Registration was achieved by the navigation system using a CT-fluoro matching technique in which preoperative CT images of the affected bone were matched to intraoperatively acquired orthoplanar (AP and lateral) fluoroscopic images (). The registration accuracy was verified by holding a navigation tracker probe to key and easily identifiable anatomical landmarks. A pneumatic tourniquet was then inflated, and the skin was incised precisely over the planned portal sites under navigation guidance. Access to the tumor cavity was achieved via cortical windows made at the portal sites, then a shoulder arthroscope (30° lens, 4 mm S.W.A.; Karl Storz, Tuttlingen, Germany) was introduced via one of the portal sites. The tumor was removed through one portal under direct magnified endoscopic visualization via the second portal. A navigation tracker, calibrated to the system, was mounted on a high-speed bone burr. Calibration ensured that the end of the burr was correctly referenced and spatially visualized in relation to the patient's anatomy (and displayed on the virtual images rendered on the navigation console) (). The entire intra-osseous surface of the tumor cavity was removed with this navigated high-speed bone burr. In addition to direct endoscopic visualization of the tumor cavity, this real-time spatial localization displayed in relation to the patient's anatomy enabled the surgeon to manipulate the burr accurately over the entire region involved by the tumor. The spatial (navigation guided) reference enabled us to confirm whether the entire affected area of bone had been curetted as planned, while the endoscope (visual guidance) enabled us to identify residual tumor on the walls of the cavity. The skeletal defect was then irrigated with hydrogen peroxide and normal saline. In four patients bone cement was injected into the defect using a cement gun, while bone substitute was used as filler in the remaining patient. All patients were allowed to resume full weight-bearing walking and immediate joint mobilization following removal of the drain 1 or 2 days after surgery.
Figure 2. The set-up for NEAT surgery. A shoulder arthroscope and high-speed bone burr mounting a navigation tracker were inserted via two working portals into the tumor cavity. Intra-lesional curettage could be performed under both direct endoscopic visualization and navigation guidance.
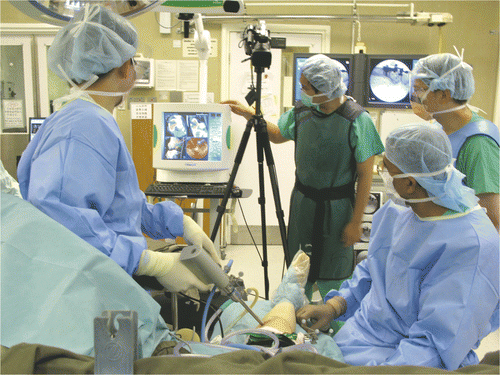
Figure 3. CT-fluoro matching was used for image-to-patient registration in Case 1. This involved the matching of preoperative CT images of a segmented bone region with two intraoperatively acquired fluoroscopic images, thus allowing minimally access registration of the preoperative CT images.
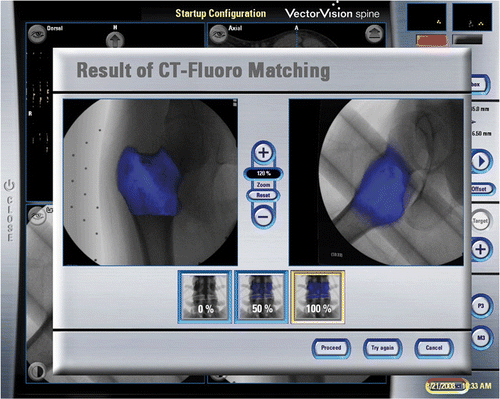
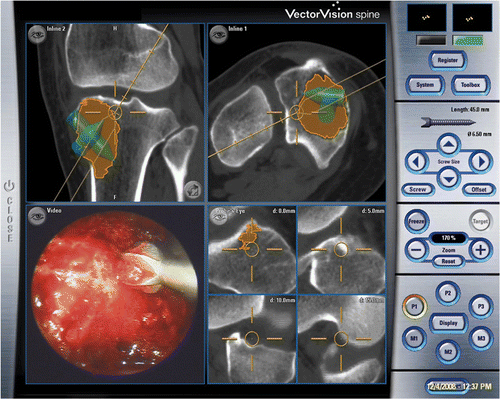
Figure 4. The tumor cavity and bone burring procedure could be directly visualized with the endoscope via small incision portal sites. The location of the navigated instrument tip (the orange circle) is shown with respect to reconstructed CT images. (The tumor is shaded in orange. The extrapolated orange line represents the direction of the instrument and is marked with 5-mm gradations, enabling it to be used to estimate the amount of bone removed and the risk of cortex perforation.)
Data recorded included the time for preoperative planning using the navigation system; the tumor volume as calculated during navigation planning; the time for operative set-up and execution of the navigation endoscopic procedures; the length of the surgical scar at the portal sites; postoperative wound pain as rated by visual analog scores (VAS); and the functional outcome.
Results
The preoperative planning for navigation took a mean of 31 min (range: 20–45 min). The mean tumor volume was 35.3 cm3 (range: 0.6–67 cm3). The mean time for navigation endoscopic tumor resection was 144 min (range: 120–165 min). The latter time depends on the actual volume of tumor to be removed, which determines the amount of burring of the tumor cavity required. The virtual preoperative CT images correlated well with the patients’ anatomy at registration. The entire tumor cavity could be curetted and burred under both direct endoscopic visualization and navigation guidance in all cases. Histology of resected tumor specimens confirmed the diagnoses in each case.
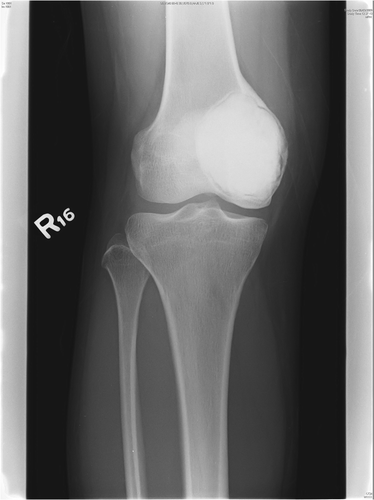
The mean wound length at each portal site was 19.5 mm (range: 15–25 mm) (). The mean early postoperative wound pain score (VAS) was 2.2 (range: 1 to 3). All patients could achieve a full range of joint movement and walk unaided at 4 weeks post-surgery. After a mean follow-up of 8.8 months (range: 7–12 months), radiological examination showed no evidence of local recurrence and good cementation of the skeletal defect ().
Discussion
This paper has described how we combined endoscopic techniques and computer assisted navigation for intra-lesional excision of benign bone tumors using a minimal access approach. The instant endoscopic observation provides instantaneous update of the progress of the surgical procedure within the cavity, supplemented with real-time visualization of the 3D skeletal anatomy and accurate spatial orientation of the operating instruments. Safe and adequate tumor clearance was thus possible, even though the access portals to the tumor cavity were small.
Extra-articular endoscopic assisted tumor resection has only been previously reported in two small case series: Futani et al. Citation[5] successfully treated one patient with bilateral calcaneal intraosseous lipomas by endoscopic assisted curettage via two small portal wounds; and Stricker Citation[4] performed an extra-articular endoscopic excision in three patients with femoral head chondroblastomas via a single portal wound. Both groups required frequent intraoperative 2D fluoroscopic imaging to verify the location of the operating tools within the tumor cavity. Neither used preoperative planning concerning the extent and volume of the tumor, nor did they plan the most optimal location of portal sites that could reach the entire tumor cavity. We found it easier and more precise to locate the portal sites by identifying the location of pre-placed virtual screws under navigational guidance during surgery. Also, the instant visual feedback of 3D images allows surgeons to obtain better and more realistic orientation than with static 2D fluoroscopic images. This technique may be particularly useful for the precise targeting of small lesions in locations where surgical access is difficult (such as the femoral neck in Case 1).
The emerging technology of CT-fluoro matching was originally designed for use in spine surgery involving CT-based navigation. Accurate matching of preoperative CT images with two intraoperative fluoroscopic images could be achieved in patients with tumors involving long bones. This technique thus enabled automatic registration of preoperative CT images without the wide surgical exposure of the bone surface that is mandatory when manual registration with CT-based navigation is required. The surgical procedures could then be performed under navigational guidance with minimal access. This registration method had advantages over that for Iso-C3D fluoroscopy as the latter requires additional radiation exposure for intraoperative image acquisition. Compared to the 3D images obtained from preoperative CT scans, images obtained intraoperatively by Iso-C3D fluoroscopy are inferior in quality and the scans cover a much smaller volume of bone Citation[10]. Therefore, automatic registration with the CT-fluoro matching technique may have great potential for executing preoperative planning with a minimal access approach in surgical navigation of musculoskeletal tumors.
As only small cortical windows were made in the affected bone after the minimal access procedure, patients were allowed to resume weight-bearing walking and joint mobilization immediately. We expect this technique may achieve faster postoperative recovery than conventional open techniques.
This study has several limitations: Our series is small, heterogeneous in diagnosis, and lacks a control group; removal of tumor through a small skin portal / bone tunnel may entail the risk of tumor implantation within the tract; and the technique requires surgeons with prior experience of both endoscopic and navigation surgery. Long-term follow-up in a larger series is required to evaluate whether the oncological and functional outcomes are indeed better or no different than with conventional approaches.
NEAT surgery combines the advantages of navigation and endoscopic techniques and has enabled intra-lesional curettage of benign bone tumors to be performed in a minimal access manner. The procedure and the tumor cavity can be clearly visualized under high-intensity illumination with a magnified endoscope. Simultaneously, the surgeon receives accurate information regarding spatial orientation in reference to the patient's anatomy which is represented in a realistic 3D manner.
It would be helpful to have dedicated navigation software for musculoskeletal tumor surgery; there is no commercially available package at present. Such a package should have the facility to integrate endoscopic images into the navigation system, and along with a special protective portal sheath for insertion of the endoscope or instruments and tools for endoscopic tumor surgery.
References
- Gunes T, Erdem M, Bostan B, Sen C, Sahin SA. Arthroscopic excision of the osteoid osteoma at the distal femur. Knee Surg Sports Traumatol Arthrosc 2008; 16(1)90–93
- Banerjee D, Eriksson K, Morris H. Arthroscopically treated intraarticular osteoid osteoma in the ankle–a report of 3 cases. Acta Orthop 2005; 76(5)721–724
- Khapchik V, O’Donnell RJ, Glick JM. Arthroscopically assisted excision of osteoid osteoma involving the hip. Arthroscopy 2001; 17(1)56–61
- Stricker SJ. Extraarticular endoscopic excision of femoral head chondroblastoma. J Pediatr Orthop 1995; 15(5)578–581
- Futani H, Fukunaga S, Nishio S, Yagi M, Yoshiya S. Successful treatment of bilateral calcaneal intraosseous lipomas using endoscopically assisted tumor resection. Anticancer Res 2007; 27(6C)4311–4314
- Wong KC, Kumta SM, Chiu KH, Antonio GE, Unwin P, Leung KS. Precision tumour resection and reconstruction using image-guided computer navigation. J Bone Joint Surg Br 2007; 89(7)943–947
- Wong KC, Kumta SM, Antonio GE, Tse LF. Image fusion for computer-assisted bone tumor surgery. Clin Orthop Relat Res 2008; 466(10)2533–2541
- Kendoff D, Hüfner T, Citak M, Geerling J, Mössinger E, Bastian L, Krettek C. Navigated Iso-C3D-based percutaneous osteoid osteoma resection: A preliminary clinical report. Comput Aided Surg 2005; 10(3)157–163
- Hwang PY, Ho CL. Neuronavigation using an image-guided endoscopic transnasal-sphenoethmoidal approach to clival chordomas. Neurosurgery 2007; 61(5 Suppl 2)212–217, discussion 217–218
- Citak M, Kendoff D, Wanich T, Pearle A, Singhai R, Krettek C, Hüfner T. The influence of distance on registration in ISO-C-3D navigation: A source of error in ISO-C-3D navigation. Technol Health Care 2006; 14(6)473–478