Abstract
Survival rates for total shoulder arthroplasty are critically dependent on the correct placement of the glenoid component. Especially in osteoarthritis, pathological version of the glenoid occurs frequently and has to be corrected surgically by eccentric reaming of the glenoid brim. The aim of our study was to evaluate whether eccentric reaming of the glenoid can be achieved more accurately by a novel computer assisted technique. Procedures were conducted on 10 paired human cadaveric specimens presenting glenoids with neutral version. To identify the correction potential of the navigated technique compared to the standard procedure, asymmetric reaming of the glenoid to create a version of −10° was defined as the target. In the navigated group, asymmetric reaming was guided by a 3D fluoroscopic technique. Postoperative 3D scans revealed greater accuracy for the eccentric reaming procedure in the navigated group compared to the freehand group, resulting in glenoid version of −9.8 ± 3.8° and −5.1 ± 4.1°, respectively (p < 0.05). Furthermore, deviation from preoperative planning was significantly reduced in the navigated group. These data indicate that our navigated procedure offers an excellent tool for supporting glenoid replacement in TSA.
Introduction
One of the most important parameters that determines the survival rate for total shoulder arthroplasty (TSA) is the proper and accurate placement of the glenoid component Citation[1]. Several reports indicate that (early) aseptic loosening of the glenoid component may occur in up to 33% of cases depending on the type of implant Citation[2], Citation[3]. A successful glenoid replacement further depends on the geometric and osseous properties of the glenoid Citation[4].
The importance of correct anatomic placement of the glenoid component in TSA is further emphasized by observations that non-anatomic placement frequently leads to eccentric loading, which predisposes the component to loosening Citation[5–7]. Anatomic studies revealed glenoid version ranging between 2° anteversion and up to 7° retroversion in the transverse plane in healthy volunteers Citation[8], Citation[9], whereas patients with osteoarthritis commonly present with an average pathological retroversion of 17° due to destruction of the posterior rim of the glenoid Citation[10].
Thus, preoperative 3D analysis of the glenoid morphology is mandatory to enable adequate preoperative planning prior to the performance of TSA Citation[11], Citation[12]. In cases of non-anatomic configuration, a physiological version of the osseous glenoid can be restored either by bone grafting or by eccentric reaming to lower the anterior rim of the glenoid Citation[13], Citation[14]. It has been suggested that a maximum of 15° retroversion can be corrected by asymmetric reaming Citation[15], Citation[16].
Several recent studies have demonstrated the feasibility of using navigation to assist glenoid and head replacement Citation[17], Citation[19]. Here, mapping of the shoulder joint was obtained from either kinematic or CT-based workflow providing real-time angulation of the instruments relative to the preoperative glenoid deformity and the humeral shaft. Recently, Habermeyer and colleagues presented the first clinical study of a CT-based navigated procedure, reporting better results with regard to placement accuracy of the glenoid component compared to the standard technique Citation[20].
The aim of our study was to assess the feasibility of a novel 3D image intensifier-based navigation system and to explore its potential for supporting eccentric reaming of the glenoid for TSA, as compared to the standard freehand technique, in a controlled, randomized experimental trial.
Methods
Study design
Ten paired human cadaver specimens preserved with Jores’ solution were randomized to either standard or navigated eccentric reaming of the glenoid. One further specimen was used as a pilot. Multiplanar views were obtained for each specimen preoperatively using a 3D image intensifier, with the glenoid version being measured in the cross-sectional plane. Guide wires leading the cannulated reamer were bilaterally inserted into the central part of the glenoidal articular surface using either the standard technique or a 3D fluoroscopic navigated procedure.
A total of 10 procedures were performed in both trial groups by the same surgeon, who is also very experienced in computer assisted surgery such that a relevant learning curve with the navigated procedure was not expected. At the end of the procedure a 3D fluoroscopic scan was again performed and the version of the glenoid surface in the cross-sectional plane was determined by a procedure-blinded investigator according to the method described by Rouleau et al. Citation[10]. Procedure time was measured and included all work steps from image acquisition to final 3D assessment.
Cadaver specimens
The cadavers presented with neutral version in the cross-sectional plane: −0.2 ± 3.4° (range: 5 to −8°) for the freehand group versus 0.6 ± 3.7° (range: 5 to −7°) for the navigated group (p > 0.05). None of the specimen showed signs of osteoarthritis, and all glenoids were classified as type A1 or A2 according to the system of Walch et al. Citation[21]. The aim of this study was to evaluate how precisely eccentric reaming of the glenoid could be performed. As the available specimens presented without pathological (retro-) version, we chose to create a glenoid with non-physiological version by asymmetric reaming and measured the accuracy of the navigated technique compared to the freehand technique. To exclude inter- and intra-observer variability, reportedly up to 4°, a glenoid version of −10° was defined as the target parameter Citation[22].
Navigated procedure
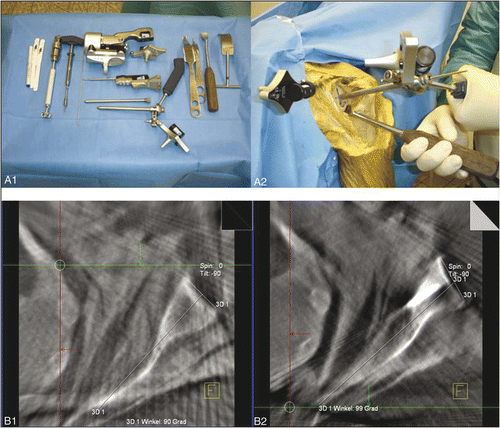
Procedures were performed on a radiolucent operating table consisting of carbon fiber-reinforced plastic (Maquet, Rastatt, Germany). After a deltoideopectoral approach with the cadaver in the beach chair position, the glenoid was reamed eccentrically with guidance from 2.8-mm wires which were inserted through a drill sleeve either freehand or with navigation. The navigated procedure was performed with the Stryker Navigation System (Stryker GmbH & Co., Duisburg, Germany) (). This navigation system is available for various trauma and orthopedic applications using kinematic or image-based workflows. Images can be obtained preoperatively from CT, MRI or an image converter.
Figure 1. Operative setting of the navigated procedure using a 3D image intensifier and a radiolucent shoulder table.
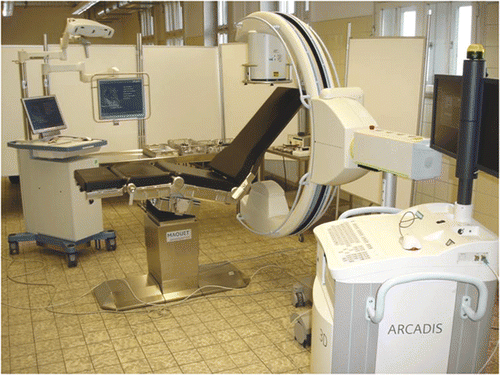
During the application, the patient, navigated instruments and C-arm have to be fitted with optoelectronic trackers. These trackers actively emit infrared signals which are detected by the camera of the navigation system, enabling intraoperative real-time tracking of instruments and the C-arm with respect to the patient's virtual anatomy. In our study, images were obtained from a 3D image converter (ARCADIS Orbic, Siemens Medical Solutions, Erlangen, Germany). Prior to the navigated procedure, the C-arm was calibrated, equipped with a tracker, and connected to the navigation system. A reference base was attached to the ipsilateral coracoid and an automated orbital scan of the shoulder joint was performed. Single C-arm images were obtained prior to the scanning to ensure that the scanned volume was correctly centered over the glenohumeral area so as to avoid the need for repetition of the scanning procedure. Images were automatically converted to a multiplanar reformatted series, which was transferred to the navigation system automatically and immediately made available for the navigated procedure without registration.
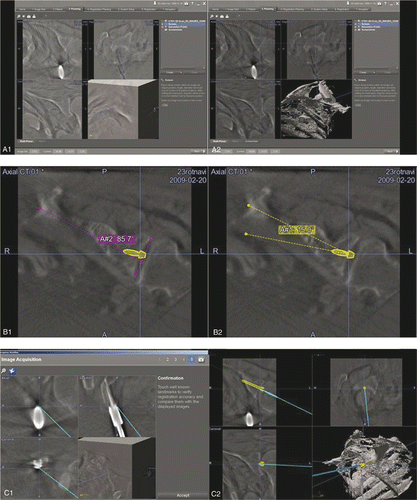
After receiving the 3D image data set, the threshold for an optimal visualization of the bone was identified and set (). After this, the preoperative planning procedure was performed. First, angulation of the glenoid in the cross-sectional plane was assessed (). Then the required screw path was identified with regard to the desired correction angle and designated as the so-called trajectory (). Before commencing the navigated procedure, the data image set was verified by using the point of entry of the reference base into the coracoid as a landmark (). The navigation system was then used to pilot the guide wire along the planned screw path ( and ). A cannulated reamer (Depuy Orthopädie GmbH, Kirkel-Limbach, Germany) was attached to the glenoid along the guide wire and eccentric reaming was performed. Finally, the glenoid version created by the eccentric reaming procedure was assessed using multiplanar reformated C-arm images, as described above.
Figure 2. Top: Preoperative threshold adjustment to bony structures (A1, A2). Center: Preoperative assessment of the glenoid version (B1) and planning of the guide wire trajectory on the navigated image data set (B2): preoperative glenoid version is 5° (pink), thus, the angle for eccentric reaming to achieve glenoid version of −10° is defined as 15° (yellow). Bottom: System verification (C1) and real-time guidance of the navigated drill sleeve along the planned trajectory (C2).
Statistical analysis
All data are presented as mean value ± standard deviation and range. Differences between the trial groups were evaluated using a standard t-test. If the sample data were not normally distributed, the Mann-Whitney rank sum test was used. All test results were calculated with a desired power of 1−β = 0.8 and a significance level of α = 0.05. Statistical analysis was performed with SigmaStat (version 2.03) (SPSS, Inc., Chicago, IL).
Results
The navigated technique revealed no technical problems and none of the procedures had to be aborted. It was found that the camera provided a sufficient operating range, and detection of the infrared signals emitted by the different trackers was excellent. Therefore, alignment and angulation of the camera required no further adjustment. Registration and calibration of the trackers was quick and feasible.
Despite the handiness of the system, the procedure time was significantly increased compared to that for the freehand technique (38.9 ± 5.6 min for the navigated technique versus 27.6 ± 5.6 min for freehand, p < 0.01). In contrast to the good feasibility of the navigation system, 3D image acquisition was found to be more challenging. The system provided a limited image window of only 12 cm3, which made it difficult to center the scanning volume correctly over the glenoid. Consequently, the preoperative scan for image acquisition had to be repeated in 4 cases (navigated and non-navigated). It was further noted that the scanning procedure was complicated in obese and/or muscular cadavers. A maximum width of approximate 55 cm from shoulder to shoulder allowed for a practicable scan.
Overall, image resolution was rather low but nevertheless allowed safe performance of navigation and reliable detection of pre- and postoperative glenoid version (). Compared to the standard technique, the navigated procedure provided greater accuracy, creating the desired glenoid version angle of −10° (). Aberrance from the planned correction angle was increased almost six-fold in the freehand group (5.8 ± 3.7° for the freehand group versus 1.0 ± 1.0° for the navigated group, p < 0.01).
Table I. Comparison of the standard freehand technique and the navigated procedure. Values are given as means and standard deviations with ranges in parentheses.
In contrast to the standard technique, the navigated procedure produced no severe divergence from the preoperative planning (). No problems resulting from bent drill bits or guide wires leading to relevant deviation occurred in either group.
Discussion
The non-navigated freehand technique is currently the standard procedure for replacement of the glenoid in total shoulder arthroplasty. Navigated assistance has been introduced for hip and knee replacement, however, and for both these joints CT-based and kinematic systems have been evaluated with good clinical results Citation[23], Citation[24]. Also, the first results have recently been reported for the use of CT-based navigation in trials on cadaveric shoulders, indicating a more precise glenoid implantation compared to that achieved with the standard technique Citation[25]. However, until now, no commercially available system has been available that is suitable for interventions in the shoulder region. Furthermore, in contrast to the hip and knee joints, quantitative methodological investigations of navigated procedures in shoulder arthroplasty remain rare.
In this paper we have presented the first results of a fluoroscopic navigated procedure to support the replacement of the glenoid. The 3D C-arm used in our study has been previously validated for navigated and non-navigated operative treatment of articular long bone fractures, the skeleton of the foot, and fractures of the thoracolumbar spine and the posterior pelvic ring Citation[26–30]. Our data indicate that the use of a 3D image converter for navigated glenoid replacement represents a feasible and safe approach. A clear limitation of this technique is that eccentric patient positioning during the scan apparently decreases the imaging quality and may make repeat scan procedures necessary. Thus, the presented navigated technique might not be applicable in all cases, especially in obese patients or those with broad shoulders.
In contrast to a CT-based procedure, use of a 3D C-arm for navigated glenoid replacement allows the surgeon to verify the operative outcome immediately, without the need for time-consuming matching procedures. An additional benefit of the C-arm in navigated procedures is that images can be obtained intraoperatively and updated at any time. Thus, in contrast to CT-based navigation, images acquired for the navigated procedure always represent the current anatomic situation and positioning of the patient. Radiation exposure was not addressed in our present study and needs to be evaluated in further investigations. The little data available suggests that, at least in spinal surgery, 3D fluoroscopic navigation is associated with a level of radiation exposure comparable to that in CT-based navigation or possibly even slightly lower Citation[31].
In summary, we suggest that the navigated procedure presented in our study offers potential benefits compared to the standard technique, especially when pathological retroversion of the glenoid can be sufficiently addressed by eccentric reaming. The experimental paradigm in this proof-of-concept study did not allow us to compare the functional outcome and survival rate of the implant devices placed with the navigated and freehand techniques. These parameters need to be evaluated in prospective randomized clinical trials in a long-term follow-up.
Overall, application of the 3D fluoroscopic navigated technique used in our study can be recommended as an excellent tool for supporting replacement of the glenoid, even though it increases the procedure time compared to the freehand method. Unresolved problems with this technique include the limited display window and variable image quality. Application of a 3D image intensifier further requires the use of radiolucent tables and an update of the navigation system, both of which are associated with significant additional costs.
Acknowledgments
The authors like to thank Depuy, Maquet, Siemens and Stryker for their technical support during the study.
Declaration of interest: The authors report no conflicts of interest related to companies or products named in the paper.
References
- Habermeyer P, Magosch P, Luz V, Lichtenberg S. Three-dimensional glenoid deformity in patients with osteoarthritis: A radiographic analysis. J Bone Joint Surg Am 2006; 88(6)1301–1307
- Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg 2009; 18(6)859–863
- Williams GR, Abboud JA. Total shoulder arthroplasty: Glenoid component design. J Shoulder Elbow Surg 2005; 14(1 Suppl S)122S–128S
- Mansat P, Briot J, Mansat M, Swider P. Evaluation of the glenoid implant survival using a biomechanical finite element analysis: Influence of the implant design, bone properties, and loading location. J Shoulder Elbow Surg 2007; 16(3 Suppl)S79–S83
- Farron A, Terrier A, Büchler P. Risks of loosening of a prosthetic glenoid implanted in retroversion. J Shoulder Elbow Surg 2006; 15(4)521–526
- Nyffeler RW, Sheikh R, Atkinson TS, Jacob HA, Favre P, Gerber C. Effects of glenoid component version on humeral head displacement and joint reaction forces: An experimental study. J Shoulder Elbow Surg 2006; 15(5)625–629
- Shapiro TA, McGarry MH, Gupta R, Lee YS, Lee TQ. Biomechanical effects of glenoid retroversion in total shoulder arthroplasty. J Shoulder Elbow Surg 2007; 16(3 Suppl)S90–S95
- Churchill RS, Brems JJ, Kotschi H. Glenoid size, inclination, and version: An anatomic study. J Shoulder Elbow Surg 2001; 10(4)327–332
- Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am 1992; 74(7)1032–1037
- Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Defranco M, Walch G. Glenoid version: How to measure it? Validity of different methods in two-dimensional computed tomography scans. J Shoulder Elbow Surg 2010; 19(8)1230–1237
- Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am 2008; 90(11)2438–2445
- Welsch G, Mamisch TC, Kikinis R, Schmidt R, Lang P, Forst R, Fitz W. CT-based preoperative analysis of scapula morphology and glenohumeral joint geometry. Comput Aided Surg 2003; 8(5)264–268
- Gillespie R, Lyons R, Lazarus M. Eccentric reaming in total shoulder arthroplasty: A cadaveric study. Orthopedics 2009; 32(1)21
- Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am 2001; 83(6)877–883
- Clavert P, Millett PJ, Warner JJ. Glenoid resurfacing: What are the limits to asymmetric reaming for posterior erosion?. J Shoulder Elbow Surg 2007; 16(6)843–848
- Nowak DD, Bahu MJ, Gardner TR, Dyrszka MD, Levine WN, Bigliani LU, Ahmad CS. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: The amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg 2009; 18(5)680–688
- Edwards TB, Gartsman GM, O’Connor DP, Sarin VK. Safety and utility of computer-aided shoulder arthroplasty. J Shoulder Elbow Surg 2008; 17(3)503–508
- Kedgley AE, DeLude JA, Drosdowech DS, Johnson JA, Bicknell RT. Humeral head translation during glenohumeral abduction following computer-assisted shoulder hemiarthroplasty. J Bone Joint Surg Br 2008; 90(9)1256–1259
- Nguyen D, Ferreira LM, Brownhill JR, King GJ, Drosdowech DS, Faber KJ, Johnson JA. Improved accuracy of computer assisted glenoid implantation in total shoulder arthroplasty: An in-vitro randomized controlled trial. J Shoulder Elbow Surg 2009; 18(6)907–914
- Kircher J, Wiedemann M, Magosch P, Lichtenberg S, Habermeyer P. Improved accuracy of glenoid positioning in total shoulder arthroplasty with intraoperative navigation: A prospective-randomized clinical study. J Shoulder Elbow Surg 2009; 18(4)515–520
- Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty 1999; 14(6)756–760
- Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: Conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg 2003; 12(5)493–496
- Beckmann J, Stengel D, Tingart M, Götz J, Grifka J, Lüring C. Navigated cup implantation in hip arthroplasty. Acta Orthop 2009; 80(5)538–544
- Matziolis G, Krocker D, Weiss U, Tohtz S, Perka C. A prospective, randomized study of computer-assisted and conventional total knee arthroplasty. Three-dimensional evaluation of implant alignment and rotation. J Bone Joint Surg Am 2007; 89(2)236–243
- Nguyen D, Ferreira LM, Brownhill JR, Faber KJ, Johnson JA. Design and development of a computer assisted glenoid implantation technique for shoulder replacement surgery. Comput Aided Surg 2007; 12(3)152–159
- Acosta FL, Jr, Thompson TL, Campbell S, Weinstein PR, Ames CP. Use of intraoperative isocentric C-arm 3D fluoroscopy for sextant percutaneous pedicle screw placement: case report and review of the literature. Spine J 2005; 5(3)339–343
- Atesok K, Finkelstein J, Khoury A, Peyser A, Weil Y, Liebergall M, Mosheiff R. The use of intraoperative three-dimensional imaging (ISO-C-3D) in fixation of intraarticular fractures. Injury 2007; 38(10)1163–1169
- Briem D, Linhart W, Lehmann W, Begemann PG, Adam G, Schumacher U, Cullinane DM, Rueger JM, Windolf J. Computer-assisted screw insertion into the first sacral vertebra using a three-dimensional image intensifier: Results of a controlled experimental investigation. Eur Spine J 2006; 15(6)757–763
- Burgkart R, Gottschling H, Roth M, Gradinger R, Schweikard A. Fluoroscopy-based 3D navigation of complex correction osteotomies at the proximal femur. Orthopade 2005; 34(11)1137–1143
- Citak M, Kendoff D, Kfuri M, Jr, Pearle A, Krettek C, Hüfner T. Accuracy analysis of Iso-C3D versus fluoroscopy-based navigated retrograde drilling of osteochondral lesions: a pilot study. J Bone Joint Surg Br 2007; 89(3)323–326
- Gebhard F, Kraus M, Schneider E, Arand M, Kinzl L, Hebecker A, Baetz L. Radiation dosage in orthopedics – a comparison of computer-assisted procedures. Unfallchirurg 2003; 106(6)492–497