Abstract
Objective: To evaluate the utility of performing endonasal transsphenoidal pituitary surgery with computer-based neuronavigation, and to examine the efficacy of computer-based neuronavigation compared to fluoroscopy.
Patients: We conducted a retrospective review of patients who underwent pituitary surgery between September 1998 and September 2008. Of 120 consecutive patients, 70 met inclusion criteria and were fully examined. The inclusion criteria were that patients had undergone endonasal transsphenoidal pituitary surgery performed by the same neurosurgeon at the same institution. Nineteen of the patients were treated using intraoperative fluoroscopy and 48 were treated using the BrainLAB VectorVision neuronavigation system. Preparation times, surgical times and associated complications were analyzed.
Results: Our results indicate that image guidance reduces the overall operating room time and complication rate. Average preparation time for fluoroscopy and computer-based neuronavigation was 70.3 and 67.3 min, respectively (p = 0.3299). Average surgical time with fluoroscopy and BrainLAB was 131 and 107.9 min, respectively (p = 0.0079). The results were also analyzed with regard to other parameters such as associated complications, age and diagnoses.
Conclusion: Computer guided endoscopic endonasal transsphenoidal surgery provides a three-dimensional image to the surgeon, allowing for greater visual accuracy and surgical precision and a faster procedure without radiation exposure or the need for additional personnel.
Introduction
The endoscopic transsphenoidal minimally invasive approach has significantly reduced morbidity and mortality in the surgical treatment of pituitary adenomas. The impressive evolution of the transnasal transsphenoidal approach has passed through several phases. The initial modest results that accompanied the development of this approach by legendary neurosurgeons such as Harvey Cushing and Jules Hardy intimidated most surgeons from venturing to use the transsphenoidal route for decades. Gerard Guiot brought about a renaissance of the transsphenoidal approach by incorporating intraoperative fluoroscopic localization, while Hardy revolutionized transsphenoidal surgery with the use of the operating microscope and microsurgery Citation[1], Citation[3]. Modernization saw the application of sophisticated technology to what were long believed to be solely manual skills such as microsurgery. More recently, the marriage of the ever-changing computer technology and intricate radiographic anatomy gave birth to neuronavigation, and CT- and MRI-guided stereotaxy has quickly replaced fluoroscopy in transsphenoidal surgery.
Traditionally, the sublabial-transeptal microscopic approach to the sphenoid has relied upon intraoperative fluoroscopy for localization and confirmation Citation[4–6]. Several advantages have been claimed for frameless stereotaxy over fluoroscopy, such as the ability of the frameless technique to provide three-dimensional (3D) orientation and real-time anatomic information to the surgeon, the absence of radiation exposure, and the shorter operative time compared to the fluoroscopic approach, which only allows only for vertical orientation. However, in the realm of evidence-based medicine, we should investigate in an objective manner that which we can clearly intuit or deduce with certainty, in order to demonstrate that new technologies do, in fact, improve outcomes and that they are cost-effective. To date, only a few studies have compared fluoroscopy to frameless transsphenoidal pituitary surgery Citation[3–10]. The goal of the present study was to evaluate the frameless method in comparison to fluoroscopy-guided endoscopic/endonasal transsphenoidal minimally invasive surgery in terms of efficacy, operative time and outcome.
Methods
Patient selection
The project was reviewed and approved by the institutional review board (IRB) of the University of South Florida and the Office of Clinical Research at Tampa General Hospital. A retrospective chart review was conducted for patients undergoing transsphenoidal pituitary surgery at the University of South Florida College of Medicine.
The database included patients who had undergone surgical treatment for their pituitary disease with image guidance between 1998 and 2008. One hundred and twenty-one consecutive patients were examined. The inclusion criteria were that the patients had undergone transnasal transsphenoidal pituitary surgery performed by the same surgeon at the same institution. Exclusion criteria were incomplete medical records for a potential subject, such as a lack of detailed operative notes or anesthesia and nursing records. Based on our original cohort, a group of 70 patients that met the inclusion criteria was obtained. Three of these cases were revision cases. Following IRB approval, age, ethnicity, gender, mode of image guidance, “in-room” time, incision time, end time, diagnosis, complications, and associated risk factors were recorded for each patient. The “in-room” time and incision times were used to calculate preparation time, while the incision time and end-time were used to calculate surgery time.
Two different imaging modalities were used during the treatment of the patients. Standard intraoperative fluoroscopy imaging was compared to frameless stereotactic computer-based neuronavigation in the form of the BrainLAB VectorVision system (BrainLAB, Feldkirchen, Germany), which is henceforth referred to as image guidance. Subsequently, patients were placed into one of three treatment arms: a fluoroscopy group; an image guidance group; and a combined fluoroscopy and image guidance group. The clinical criteria pooled from the database were then compared across these groups.
Preoperative imaging
For frameless stereotaxy, patients underwent preoperative non-contrasted CT scanning of the sinus and skull base, which calls for fine-cut (1–2 mm) axial CT scans. The imaging data was then transferred via CD-ROM, flash drives, or computer network to the operating room and loaded into the workstation for automatic reconstruction in the coronal and sagittal planes.
Neuronavigational image guidance system
The BrainLAB VectorVision system has been previously described elsewhere Citation[4–6], Citation[9]. In summary, it is a Windows-based two-camera operating system in which infrared light is emitted by transmitters installed near the receiving cameras and reflected by localizers or markers. These reflections are detected and used by the computer to establish both the positional coordinates of the patient's head and also the position of surgical instruments.
All patients were positioned with three-point head fixation in a Mayfield clamp, and a reference star array was attached adjacent to the clamp. After the navigation system was positioned next to the monitor displaying the endoscopic view, surface registration of the patient was carried out using Z-Touch laser registration Citation[4], Citation[6], Citation[11], Citation[12]. A virtual model of the patient's facial surfaces was then created by the computer, correlating with the facial surface provided by the CT imaging data. Following completion of this matching process, the navigation system gave an estimated value of the approximation of the computer-generated point and the actual point measured on the patient. Anatomical landmarks on the patient's face (i.e., the lateral and medial canthi, tip of the nose, columella, nasion, maxillary teeth, anterior wall of the sphenoid sinus, etc.) were verified by a marked pointer instrument in reference to the corresponding position provided by the navigation system, thereby confirming the precision of the system.
Operative technique
The endonasal transsphenoidal approach has been previously described in detail elsewhere Citation[1], Citation[13]. Generally speaking, the approach to the anterior face of the sphenoid follows the paraseptal passageway, medial to the middle turbinate, allowing the remaining sinuses lateral to the middle turbinate to be left undisturbed. When necessary, gentle lateralization of the middle turbinate is done with a Freer elevator. The sphenoid ostium is identified in the sphenoethmoid recess, at the level of the inferior edge of the superior turbinate. The sinus is entered and the ostium enlarged in an inferior and medial direction. An inferiorly based mucoperiosteal flap is raised off of the posterior bony septum, which is debrided to expose the contralateral sphenoid ostium. The face of the sphenoid sinus is then opened widely to expose the sinus and sella medial to the left and right ostia.
Statistical analysis
Univariate and multivariate analysis of the relationship of clinical variables was undertaken. The t-test and Fisher's exact test were used to compare group proportions. The level used to determine statistical significance was a p-value below 0.05. For univariate analysis, the t-test and Fisher's exact test were performed while only one variable was in the model without controlling for other potential factors. To compare the differences in the demographic information, surgery time, preparation time, diagnosis, and adverse events between the fluoroscopy and image guidance groups, a logistic regression model was used.
Adverse events
The adverse events comprised complications observed during or after completion of surgery. Diagnoses of residual tumor, CSF leak, diabetes insipidus, or hemorrhage were made based on review of the charts. Furthermore, residual tumor was defined as incomplete resection based on postoperative imaging and/or the need for repeat surgery. CSF leak was defined as having been diagnosed from intraoperative visualization, positive postoperative CSF rhinorrhea, and/or a postive beta transferrin test. Diabetes insipidus and hemorrhage were diagnosed on the basis of clinical parameters and uncontrolled bleeding, respectively.
Results
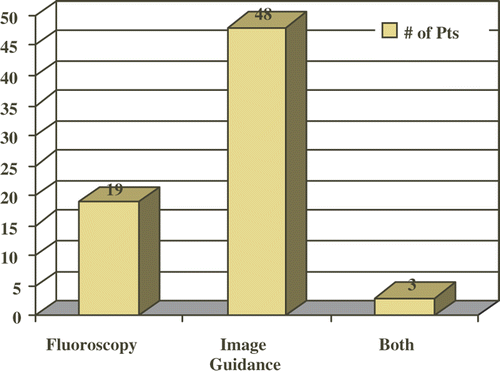
One hundred and twenty-one consecutive patients underwent image guided minimally invasive pituitary surgery. Of these, 70 patients qualified for inclusion in the study based on the inclusion and exclusion criteria. Nineteen cases (27%; mean age 44.2 years) involved fluoroscopy only, 48 cases (69%; mean age 54.5 years) involved image guidance, and 3 cases (4%; mean age 36.3 years) involved both fluoroscopy and image guidance (). The patients in the image guidance group were significantly older (p = 0.0208). The majority of the patients were Caucasian, and there was no statistically significant difference between the groups with respect to ethnicity (p = 0.8141). There were 10 females (53%) and 9 males (47%) in the fluoroscopy group and 28 females (58%) and 20 males (42%) in the image guidance group (p = 0.7861). There was an overwhelming preponderance of macroadenoma as the main pathologic diagnosis in the image guidance group, with 40 cases (83%) vs. only 9 (47%) in the fluoroscopy group (47%) (p = 0.0028). Other diagnoses included craniopharyngioma, cystic adenoma, microadenoma, and Rathke's cleft cyst.
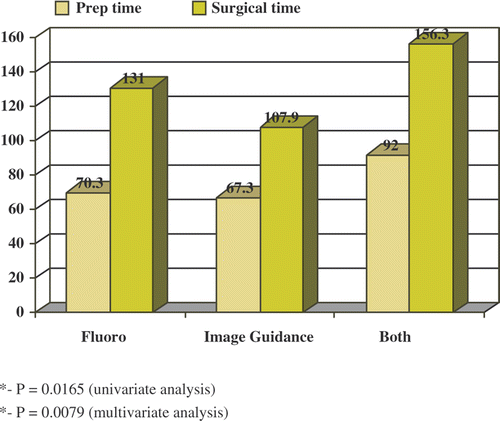
Although there was a reduction in mean preparation time in the image guidance group (67.3 min) compared to the fluoroscopy group (70.3 min), univariate analysis (, ) shows that this difference in preparation time was not significant between the two groups (p = 0.6577). However, surgery time was significantly shorter (, ) in the image guidance group (107.9 min) than in the fluoroscopy group (131 min) (p = 0.0165).
Table I. Univariate analysis.
Multivariate analysis () shows that after controlling for types of diagnosis and age, the difference in preparation time was not significant between the two groups (p = 0.3299 by the linear regression model). However, after controlling for the types of diagnosis and age, the linear regression model shows the surgery time to be significantly shorter () in the computer-based neuronavigation group than in the fluoroscopy group (p = 0.0079). Note that the three patients in the combined group () show increased preparation and surgical time of 92 and 156.3 min, respectively. However, because of the small sample size, no statistical analysis is applicable for the combined group. This group represents the transition period from fluoroscopy use to exclusive use of image guidance and is included to represent our learning curve.
Table II. Multivariate analysis.
A total of 30 patients incurred adverse events, for an overall rate of 45%. The proportion of patients affected with adverse events ( and ) was higher in the fluoroscopy group with 12 of 19 patients (63.2%) compared to only 18 of 48 patients (37.5%) in the computer-based neuronavigation group. The Fisher's exact test shows that the number of patients thus affected in the fluoroscopy group was of marginal significance compared to the computer-based neuronavigation group (p = 0.0507). However, after controlling for age and diagnosis, this difference becomes insignificant (p = 0.263). In addition, a total of 35 complications occurred in this series, with an overall complication rate of 52%. One patient in the fluoroscopy group experienced two simultaneous events, while three patients in the image guidance group experienced two simultaneous events. Therefore, there were 13 (68%) complications in the fluoroscopy group and 22 (46%) complications in the image guidance group. This difference in complication rate between the two groups was not statistically significant (p = 0.0805). In addition, no statistical significance was noted between groups for specific complications ().
Table III. Adverse events.
Discussion
Pituitary surgery has witnessed tremendous modification as the evolution process continues. The minimally invasive transnasal endoscopic approach has now replaced the traditional sublabial/transseptal microsurgical approach Citation[1], Citation[2]. However, endoscopes provide only a 2D representation in a complex 3D space, along with perceptual distortion secondary to the fish-eye effect, which may lead to surgical errors. In the age of sophisticated technology, image guided surgery has brought pituitary surgery to its prime. Intuitively, computer-based image guidance should offer a safer, more precise, and more efficient technique compared to standard intraoperative fluoroscopy.
A thorough knowledge of anatomical variances of the sphenoid bone/sinus and precise orientation while approaching the sella are key to successful transsphenoidal surgery. Maintaining the appropriate trajectory, especially in revision cases, is imperative for avoiding complications in light of the proximity of the sella to the optic nerves, internal carotid arteries, cranial nerves and hypothalamus. Deviations within the sagittal plane can potentially lead to vascular, endocrine, visual, neurological and intracranial complications. Although image guided surgery has become a crucial part of pituitary surgery, objective data to support its value have been only sparsely reported Citation[14]. Our goal in this study was to objectively evaluate the role of computer-based neuronavigation (using the BrainLAB VectorVision) as a practical means of image guidance and help justify its current applications as a standard tool in transsphenoidal pituitary surgery.
Clinical impact of image guidance
Our results indicate that computer-based neuronavigation (image guidance) reduces overall operating room time, contrary to previous reports in the literature Citation[5], Citation[6]. Mean preparation time was reduced from 70.3 min using fluoroscopy to 67.3 min using image guidance (p = 0.6577 univariate analysis; p = 0.3299 multivariate analysis). In addition, operative time was significantly reduced from 131 min using fluoroscopy to 107.9 min using image guidance (p = 0.0165 univariate analysis; p = 0.0079 multivariate analysis). Also, our overall reduced rate of complications when using image guidance () approached near significance (p = 0.0507), which may correlate with safer surgeries. However, our incidence of CSF leaks was higher in the image guidance group (where CSF leak was defined as documentation of visualization of CSF intraoperatively and/or diagnosis postoperatively). We may attribute this finding to more aggressive resection when using image guidance. Also, three of these image guided cases were revision cases.
Advantages over fluoroscopy
In contrast to fluoroscopy, image guidance provides continuous imaging. This obviates the requirement for radiological personnel to operate the C-arm fluoroscopy device intraoperatively. In addition, by eliminating fluoroscopy from the procedure, the risk of radiation exposure for the surgeon, staff and patient is reduced while avoiding the need for cumbersome lead aprons throughout the surgery Citation[15], Citation[16]. The foremost limitation of fluoroscopy is the 2D imaging, which provides sagittal guidance only; with image guidance, multiplanar 3D images are continuously accessible. Furthermore, clinical and cadaveric studies have verified the reliability and accuracy of various computer-based systems Citation[7–10], Citation[17].
Disadvantages of image guidance
There are still a few drawbacks to the use of computer-based image guidance in pituitary surgery. All data is acquired preoperatively, therefore neuronavigation cannot reflect morphological changes produced during surgery Citation[9]. Additionally, image guidance requires CT imaging with fine, thin axial cuts through the skull base and paranasal sinuses, which in itself requires no additional cost, time, or personnel; however, by the time the patient reaches the specialized consultant, a second imaging procedure may be required in addition to the primary diagnostic study, thereby increasing both radiation exposure and costs.
Limitations of the study
The limitations of our study are as follows. First of all, it is a retrospective review. Moreover, the study does not take into account the associated clinical risk factors seen in our presenting patients that may impact surgical outcomes, such as preoperative and postoperative hormonal considerations, pituitary apoplexy, acromegaly, Cushing's disease, preoperative vision changes, or revision surgery. Also, we recognize the small sample size and imbalance in our patient populations, with only 19 cases having used fluoroscopy compared to 48 cases using image guidance. This discrepancy in sample size reflects the retrospective nature of the study and highlights the shift toward image guidance from intraoperative fluoroscopy. The American Academy of Otolaryngology – Head & Neck Surgery (AAO-HNS), in its published policy statement on intraoperative use of computer aided surgery, acknowledged that it would be impossible to support the use of such technology with level I evidence Citation[18]. We consider our limited retrospective analysis a step in the right direction of evidence-based medicine.
Overall, our findings translate into more efficient and safer surgeries, reducing the morbidity associated with pituitary surgery. As one outcome, we now use computer-based neuronavigation image guidance exclusively in our endoscopic approach to the sphenoid in pituitary surgery. The main points of criticism of computer-based imaging have been the increased cost, prolonged set-up and increased operative time Citation[5], Citation[6], and our results clearly refute these concerns. Intuitively, image guidance provides superior localization and confirmation of critical landmarks, providing a means for a faster approach and resection of sellar lesions.
Acknowledgments
We would like to thank Ren Chen, MD, MPH, in the Office of Research for providing statistical assistance.
References
- Senior BA, Ebert CS, Bednarski KK, Bassim MK, Younes M, Sigounas D, Ewend MG. Minimally invasive pituitary surgery. Laryngoscope 2008; 118: 1842–1855
- Abou-Jaoude PM, Zeitouni AG, Soualmi L, Leblanc R. Multimodal multidisciplinary surgical approach for the treatment of pituitary tumours. J Otolaryngol 2007; 36: 322–326
- Asthagiri AR, Laws ER, Jane JA. Image guidance in pituitary surgery – a modern approach. Pituitary Surg 2006; 34: 46–63
- Stelter K, Andratschke M, Leunig A, Hagedorn H. Computer-assisted surgery of the paranasal sinuses: Technical and clinical experience with 368 patients, using the VectorVision Compact system. J Laryngol Otol 2006; 120: 1026–1032
- Charalampaki P, Reisch R, Ayad A, Welschehold S, Conrad J, Wüster C. Image-guided endonasal transsphenoidal microsurgical treatment of recurrent microadenomas of the pituitary gland. Minim Invasive Neurosurg 2006; 49: 93–97
- Elias WJ, Chadduck JB, Alden TD, Laws ER, Jr. Frameless stereotaxy for transsphenoidal surgery. Neurosurgery 1999; 45: 271–275, discussion 275–277
- Lasio G, Ferroli P, Felisati G, Broggi G. Image-guided endoscopic transnasal removal of recurrent pituitary adenomas. Neurosurgery 2002; 51: 132–137
- McCutcheon IE, Kitagawa RS, Demasi PF, Law KB, Friend KE. Frameless stereotactic navigation in transsphenoidal surgery: Comparison with fluoroscopy. Stereotact Funct Neurosurg 2004; 82: 43–48
- Zhao Y, Yu S, Wang R, Zhao J. Clinical application of neuronavigation system in transsphenoidal surgery of pituitary macroadenoma. Neurosurg Rev 2006; 29: 306–311, discussion 311–312
- Gong J, Mohr G, Vezina JL. Endoscopic pituitary surgery with and without image guidance: An experimental comparison. Surg Neurol 2007; 67: 572–578
- Greenfield JP, Howard BM, Huang C, Boockvar JA. Endoscopic endonasal transsphenoidal surgery using a skull reference array and laser surface scanning. Minim Invasive Neurosurg 2008; 51: 244–246
- Ledderose GJ, Stelter K, Leunig A, Hagedorn H. Surface laser registration in ENT-surgery: Accuracy in the paranasal sinuses – a cadaveric study. Rhinology 2007; 45: 281–285
- Snyderman CH, Carrau RL, Kassam AB. Endonasal approach to the sella and parasella areas. Operative otolaryngology: Head and neck surgery., 2nd, EN Meyers, DE Eibling. Saunders Elsevier, Philadelphia, PA 2008; 1045–1051
- Jagannathan J, Prevedello DM, Ayer VS, Dumont AS, Jane JA, Jr, Laws ER. Computer-assisted frameless stereotaxy in transsphenoidal surgery at a single institution. Neurosurg Focus 2006; 20(2)E9
- Nauer CB, Eichenberger A, Dubach P, Gralla J, Caversaccio M. CT radiation dose for computer-assisted endoscopic sinus surgery: Dose survey and determination of dose-reduction limits. Am J Neuroradiol 2009; 30: 617–622
- Ulmer S, Schulz E, Moeller B, Krause UR, Nabavi A, Mehdorn HM, Jansen O. Radiation dose of the lens in trans-sphenoidal pituitary surgery: Pros and cons of a conventional setup using fluoroscopic guidance and CT-based neuronavigation. Am J Neuroradiol 2007; 28: 1559–1564
- Carvi y Nievas MN, Höllerhage H. Reliability of neuronavigation-assisted trans-sphenoidal tumor resections. Neurol Res 2007; 29: 557–562
- American Academy of Otolaryngology-Head and Neck Surgery. The AAHNS Practice and Advocacy Page. http://www.entnet.org/Practice/policyintraOperativeSurgery.cfm