Abstract
In the field of hip resurfacing arthroplasty, accurate femoral component placement is important to achieving a positive outcome and implant survival in both the short and long term. In this study, femoral component placement was defined preoperatively using virtual computed tomography-based surgical simulation of a classical posterior surgical approach. Custom-made surgical drill guides were produced to reproduce the surgical plan in the operating room. We first developed a custom-made guide for guide-wire placement to position the femoral resurfacing component. Then, to assess the accuracy in vivo, the custom-made guide was evaluated in five patients with normal anatomy. The first hypothesis of this patient study was that the use of custom-made neck guides would allow for an average accuracy within the range of ±4° for the drill path and ±4 mm for the entry point of the guide-wire. A second hypothesis was that three-dimensional preoperative planning would enable the prediction of an implant size differing by a maximum of one size from the size eventually implanted.
The presented hip resurfacing guide performed well in terms of fit, stability and accuracy. The in vivo accuracy study revealed an accuracy of 4.05 ± 1.84° for the drill path and 2.73 ± 1.97 mm for the entry point of the guide-wire. The predicted component sizes and the implanted component sizes differed maximally by one size, confirming our hypothesis. We conclude that these preliminary data are promising, but require further validation in a full clinical setting in larger patient groups.
Introduction
Hip resurfacing arthroplasty is recognized as a valuable surgical option that is particularly suited to the young hip patient who wants to resume a fully active lifestyle after surgery. Hip resurfacing is a novel technique with a substantial learning curve Citation[1], the surgical procedure being both complex and technically demanding. Significant differences have thus arisen with evolving surgeon experience concerning intraoperative femoral component placement Citation[2]. Accurate femoral component positioning has been shown to be critical to avoiding early implant failure Citation[3], to reducing the risk of component malalignment and notching, which are the major causes of femoral neck fracture Citation[4], and to reducing the risk of metal ion release Citation[5]. The methods used conventionally to place the femoral component depend on a standard mechanical drill guide, two-dimensional templating and surgeon experience. However, the standard drill guide can produce significant variability in implant placement Citation[6]. Other technologies currently in use are navigation systems Citation[6].
Alternative solutions have been investigated with a view to decreasing the risk of implant placement outliers in hip resurfacing Citation[7]. The primary purpose of this study was to develop a custom-made guide, designed and manufactured by Materialise NV (Heverlee, Belgium), for guide-wire placement to position the femoral component in hip resurfacing through a classical posterior approach. The accuracy of the guide was then determined in vivo. As a reference for the measured accuracy we used average accuracy values for the use of computer-assisted guidance as reported in the literature. Depending on the type of angle measured and the approach used, values have been reported ranging from 2.4° Citation[8] to 8° Citation[9]. Our first hypothesis was that the use of custom-made surgical guides would allow for an average accuracy within the range of ±4° for angular deviation of the pin and ±4 mm for the entry point. Our second hypothesis was that three-dimensional (3D) preoperative planning would enable the prediction of an implant size differing by a maximum of one size from the size eventually implanted.
Materials and methods
The following sub-sections describe the materials and methods in three stages. The first describes the general process for performing the surgical planning and producing the guide; the second explains the guide development approach employed; and the third describes how the accuracy of the guide was assessed.
Computer aided surgery and surgical approach
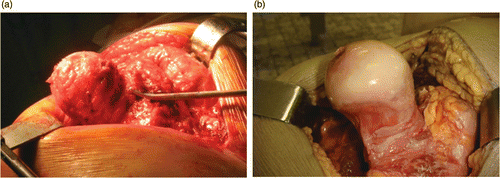
To perform the virtual surgical planning for an individual case, a preoperative CT scan was acquired according to the following scan protocol parameters: slice increment between 1 and 2 mm, 512×512 resolution, and scan field of view from the femoral head to the lesser trochanter. The scan data were converted into DICOM format and imported into the Mimics® medical image-processing software (Materialise NV, Heverlee, Belgium). A gray-value-based segmentation enabled the creation of a virtual 3D reconstruction of the femoral bone surface () according to the optimal parameter settings as defined by Gelaude et al. Citation[10].
Figure 1. Computer aided surgery procedure. Segmentation of the bony region (a) is followed by virtual component placement (b) (image created using Mimics®).
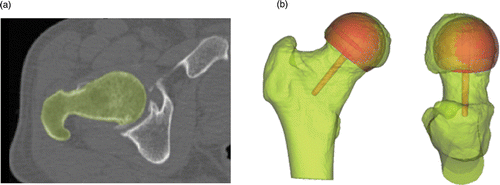
To perform the planning, a generic femoral implant component was loaded into the Mimics® software package. The visualized component allowed the surgeon to make the necessary decisions regarding the implant orientation and size ().
Based on the implant orientation as chosen during this virtual planning process, a custom-made drill guide was designed preoperatively and produced by Materialise NV using a selective laser sintering technique. The guide is manufactured from PA 2200, a polyamide material cleared by the US Food and Drug Administration. The parts were cleaned ultrasonically, and quality control was performed by optical scanning using an Atos2 scanning device (GOM Intl. AG, Wilden, Switzerland). Vapor sterilization was performed in the clinical facility according to a standard cycle for instrumentation (steam sterilization for 20 min at 121°C).
The intervention was performed by two experienced orthopaedic surgeons (E.A. and C.P). The cadaver specimen was positioned in the lateral decubitus and a classical Moore/Southern posterior approach to the hip was performed. After surgical dislocation, the custom-made drill guides were applied to the femoral head and neck with a sliding movement. The direction provided by the drill guide was used to insert a 3.2-mm Kirschner guide-wire into the bone. The subsequent surgical intervention was carried out as per the standard protocol for hip resurfacing.
Postoperatively, a quantitative analysis of the achieved drill direction was performed. To this end, CT scans were acquired and 3D models of the bone and implant direction were assessed. The postoperative bone models were matched to the preoperative bone models using the 3-matic® software (Materialise NV) Citation[11]. 3-matic® is a software package that allows common Computer Aided Design (CAD) operations on 3D anatomical data (Mimics®). The ability to use CAD operations on anatomical data permits patient-specific design of implants and devices. Deviations between the planned and actual drill directions were measured as the 3D angular deviation between these two directions and the Euclidean distance between the planned and postoperatively determined insertion points of the drill into the femoral head.
Development of custom-made guide: Iterative approach
An iterative approach was used to develop a custom-made hip resurfacing guide using six hips (from three cadavers). The cadaver specimens were all male, though the age and previous medical history were unknown. Prior to use of the specimens in the present study, disorders of the hip joint were excluded by CT imaging. For the development of the custom-made hip resurfacing guides several surgical and technical requirements were considered, of which the most important were a unique fit and proper stability. The development process consisted of four main phases.
In the initial guide approach phase, the goal was to determine the supportable bony areas, given the limitations in surgical exposure through a posterolateral surgical approach, and surgeon preferences. This initial cadaver study resulted in a set of constraints.
Secondly, an iteration process of concept development, testing and selection was performed. The concept development phase was designed to generate multiple concepts for hip resurfacing guide-wire placement, taking into account the set of constraints from the first phase. This produced a set of guide concepts, which were tested qualitatively in the concept testing phase. Qualitative feedback on design improvements was gathered and each guide concept was scored on the most important requirements. In the concept selection phase, the best performing guide concepts were selected to proceed to the next iteration. Based on the qualitative scoring, an initial selection of guides was made to undergo drilling and postoperative scanning. A second selection was made based on the quantitative scoring after postoperative analysis, comparing the planned guide-wire position with the actual drilled position. shows this quantitative scoring. In order to proceed to the next iteration, the guide concepts had to have been scored as acceptable, good or very good.
Table I. Quantitative score table for guide concepts – 3D measurement (absolute values).
Thirdly, based on the results from previous phases, the final concept was selected in the concept finalization phase.
Fourthly, the goal of the concept optimization phase was to optimize the size of the different components of the guide. The result was a so-called design freeze where the guide geometry was set.
Clinical study of guide accuracy
To assess the accuracy of the guide, a five-patient study was set up and approval from the Ethics Committee was obtained. The five male patients were selected consecutively and informed consent was obtained from each of them. All five patients presented with idiopathic early osteoarthrosis of the hip. Their ages ranged from 33.6 to 56.8 years (mean: 51.3 years). In all cases the CONSERVE® PLUS resurfacing device was implanted (Wright Medical Technology, Memphis, TN).
The guide was tested qualitatively by feedback delivered by the surgeons and a quantitative analysis was performed as described above. The implant size was determined preoperatively based on the anatomy of the bone and compared with the actually implanted size.
Results
Main components of guide
Compared to multiple guides designed for the same purpose, the optimized guide design performed well in terms of fit, stability and accuracy. Preliminary results from the cadaver study revealed the following angular deviations between the planned and actually drilled guide-wire for the described guide: 0.56°, 2.07° and 1.92°. The corresponding entry point distance differences were 1.41 mm, 2.17 mm and 1.80 mm, respectively.
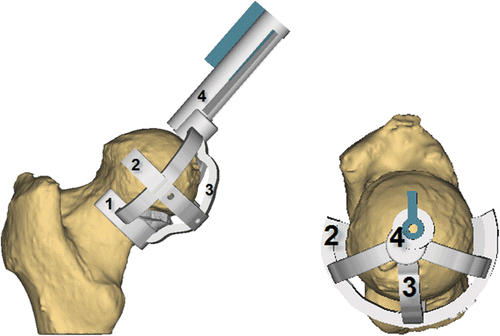
In a classical posterior surgical approach, the hip resurfacing drill guide is intended to be applied on the medial side of the bone. depicts the final guide design. The guide features the following components: a neck collar, a knife collar, a guiding cylinder and connecting arms. The neck part was designed to fit the bony part of the neck and head region of the femur to preserve the superior soft tissues and blood supply. A number of sharp contacts were added to cut through the femoral cartilage and provide stability to the guide. A drilling mechanism was created to ensure easy removal of the guide from the bone after the guide-wire has been drilled. Connecting arms keep the other components together and provide strength to the guide.
Postoperative analysis
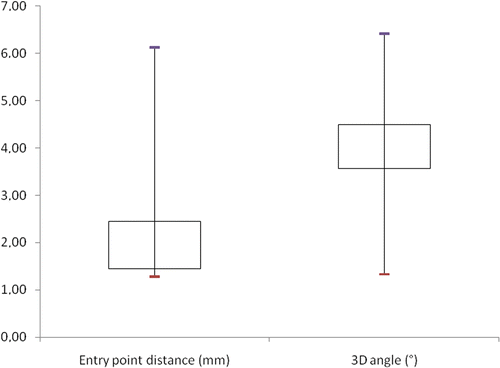
No guide-related complications occurred during surgery, and all patients were rehabilitated according to the standard procedures. All patients were scanned with the implant in place and the actual guide-wire position was compared to the planned position. The in vivo accuracy study revealed an accuracy of 4.05 ± 1.84° for the drill path and 2.73 ± 1.97 mm for the entry point of the guide-wire. shows a box plot of the 3D angle deviations and distance deviations. Comparing the predicted and actual implant sizes, a one-size difference was noted in all cases.
Discussion
Computer aided surgery and surgical approach
The computer aided surgery process described in this paper was performed based on CT images. The use and feasibility of low radiation protocols and MRI for this application will be investigated in the future. Issues relating to the inclusion of osteophytes and calcified soft tissue in the bone model are not always obvious, since the intraoperative situation is not always known beforehand. Osteophytes can be scratched away during surgery, but this may not always be desirable. Calcified soft tissues may either collapse during surgery, or be very tough and difficult to remove. Furthermore, the soft tissue on the neck is preferably preserved. For this study, decisions were made on a case-by-case basis; further work is necessary to obtain an optimal decision process.
When performing the surgical planning on the 3D models, we did not focus on creating a generic optimal planning, but rather on reproducing the preoperative planning in the operating room. Further research is required to determine the optimal femoral implant position in a repeatable manner.
Fit of custom-made guide
The presented drill guide is custom-made and allows reproduction of a virtual hip resurfacing surgical planning in the operating room. The guide was designed to obtain a unique fit and stability. It further permits verification of the correctness of the fit by visual inspection of the neck and knife contact regions. When a correct fit is found, conformably good results are expected: in our quantitative scoring system, the preliminary value of 0.56° reported for the cadavers was classified as “very good”. Consequently, the custom-made guide offers a valuable alternative to standard instrumentation and navigation to reduce the variability of implant placement in hip resurfacing. Furthermore, it has the potential to reduce the learning curve in hip resurfacing in a similarly manner to navigation systems Citation[3]. The surgeons participating in this study commented that, compared to navigation systems, the guide saved time since no extra processes (system set-up, instrument calibration, etc.) were required. However, in view of the limited number of patients in the present study, this should be investigated further.
Accuracy of custom-made guide
The mean 3D angular deviation of 4.05° is close to our acceptance range of ±4°. The maximum deviation of 6.42° was better than the maximum average value of 8° Citation[9] reported for navigation. The mean distance deviation of 2.73 mm falls within our acceptance range. The difference between the predicted and implanted sizes was one size at maximum, confirming our hypothesis. The difference between the accuracy results for the cadavers and the patients was most probably caused by differences in the amount of soft and hard tissue (), e.g., the presence of a viable capsule and osteophytes. Other parameters considered to influence the accuracy are segmentation errors, manual instrument handling errors, guide-wire elasticity and instrument variability. These should be investigated further in a larger patient group.
Conclusion
Using an iterative process, a novel CT-based guide for hip resurfacing has been developed for use with a classical posterior approach. The guide enables a unique fit and good stability to be obtained. The average 3D angular deviation of 4.05° is close to our acceptance range of ±4°. Comparing these initial results to published results for computer-assisted hip resurfacing indicates that custom-made guides have a superior performance; however, a larger patient group is required for further evaluation.
Declaration of interest: The study has been supported by Materialise N.V.
References
- Witjes S, Smolders J, Beaulé P, Pasker P, Van Susante J. Learning from the learning curve in total hip resurfacing: A radiographic analysis. Arch Orthop Trauma Surg 2009; 129(10)1293–1299
- Romanowski J, Swank M. Imageless navigation in hip resurfacing: Avoiding component malposition during the surgeon learning curve. J Bone Joint Surg Am 2008; 90(1)65–70
- Seyler T, Lai L, Sprinkle D, Ward W, Jinnah R. Does computer-assisted surgery improve accuracy and decrease the learning curve in hip resurfacing? A radiographic analysis. J Bone Joint Surg Am 2008; 90(1)71–80
- Davis ET, Olsen M, Zdero R, Waddell JP, Schemitsch EH. Femoral neck fracture following hip resurfacing: The effect of alignment of the femoral component. J Bone Joint Surg Br 2008; 90(11)1522–1527
- Williams S, Leslie I, Isaac G, Jin Z, Ingham E, Fisher J. Tribology and wear of metal-on-metal hip prostheses: Influence of cup angle and head position. J Bone Joint Surg Am 2008; 90(3)111–117
- Hodgson AJ, Inkpen KB, Shekhman M, Anglin C, Tonetti J, Masri BA, Duncan CP, Garbuz DS, Greidanus NV. Computer-assisted femoral head resurfacing. Comput Aided Surg 2005; 5(10)337–343
- Raaijmaakers M, Gelaude F, De Smedt K, Clijmans T, Dille J, Mullier M. A custom-made guide-wire positioning device for hip surface replacement arthroplasty: Description and first results. BMC Musculoskeletal Disorders 2010; 11: 161–166
- Belei P, Skwara A, de la Fuente M, Schkommodau E, Fuchs S, Wirtz DC, Kamper C, Radermacher K. Fluoroscopic navigation system for hip surface replacement. Comput Aided Surg 2007; 12(3)160–167
- Davis ET, Gallie P, Macgroarty K, Waddell JP, Schemitsch E. The accuracy of image-free computer navigation in the placement of the femoral component of the Birmingham Hip Resurfacing: A cadaver study. J Bone Joint Surg Br 2007; 89(4)557–560
- Gelaude F, Vander Sloten J, Lauwers B. Accuracy assessment of CT-based outer surface femur meshes. Comput Aided Surg 2008; 13(4)188–199
- Besl P, McKay N. A method for registration of 3-D shapes. IEEE Trans Pattern Anal Machine Intel 1992; 14(2)239–254