Abstract
Objective: Optic nerve sheath meningiomas (ONSMs) represent the most challenging lesions involving the optic pathways: Microsurgery is not indicated and classical single-stage radiosurgery appears to be too risky due to the expected destruction of the common blood supply with consequent loss of vision. Staged radiosurgery might be one treatment option because it exploits the ability of normal tissues to repair sub-lethal radiation-induced damage, offering a chance to control tumor growth while sparing function. Staged robotic radiosurgery was offered to 5 patients harboring ONSMs with the aim of sparing vision while achieving local growth control.
Patients and Methods: Five patients with ONSM presenting with visual field deficits and loss of visual acuity were treated with staged CyberKnife radiosurgery, receiving 20 Gy in 4 stages (5 Gy per stage). Treatment planning was based on contrast-enhanced thin-slice CT (1.25 mm thickness for the first three cases, 0.5 mm for the last two) and volumetric MR imaging (1.5 T for the first three cases, 3 T for the last two). An interval of 24 hours was strictly observed between stages. Visual acuity and visual fields were assessed in all patients immediately prior to treatment and at intervals of 6 months thereafter. Follow-up MRIs were performed every 6 months for 2 years, then once per year.
Results: The entire procedure, inclusive of imaging, treatment planning and treatment delivery, was performed in 5 days. Irradiation required approximately 45 min per stage. Mean tumor volume was 2.94 cc (range: 0.8–6.4 cc). Treatment was well tolerated in all patients. Follow-up ranged from 36 to 74 months. Local growth control was achieved in all patients. Restoration of normal vision was experienced by 4 patients 6 to 12 months after the treatment. One patient, who was also affected by diabetic retinopathy, showed a modest improvement after 6 months, remaining stable thereafter.
Conclusion: Staged CyberKnife radiosurgery provides a fast and well-tolerated non-invasive treatment with excellent visual outcomes. If these preliminary results are confirmed by larger series, staged radiosurgery could be proposed as a first-line treatment for ONSM.
Introduction
Optic nerve sheath meningiomas (ONSMs) are rare lesions, representing approximately 1 to 2% of all intracranial meningiomas, but their origin and location make them one of the most demanding treatment challenges in neurosurgery. Primary ONSMs originate from the meningeal sheath of the orbital or canalicular segment of the optic nerve. Secondary ONSMs have an intracranial origin and invade the orbit, compressing and displacing the optic nerve. This paper reports a new treatment option for primary ONSM and will therefore refer exclusively to this entity. Primary ONSMs share the same pial blood supply as the optic nerve itself: both microsurgical resection and stereotactic irradiation are therefore likely to injure the nerve's blood supply, leading to blindness. ONSMs are most commonly found in middle-aged women, but may also occur in children with neurofibromatosis type II, in whom they display a more aggressive growth pattern sometimes involving both optic nerves. They are typically detected when patients present with mild proptosis or progressive, painless loss of visual acuity or visual field, most often peripheral constriction. Their growth pattern has classically been viewed as typically circumferential along the optic nerve, although it is not uncommon to find lesions displacing the nerve peripherally (this could be an initial stage of the disease, followed over time by encasement of the nerve). The symptoms are those of a compressive neuropathy and include, in the early stages, dyschromatopsia and afferent pupillary defects. At the time of diagnosis the patients present with more or less marked visual field deficits, reduced visual acuity, and optic disc edema. Over time, ONSMs may extend through the optic canal intracranially. Optic chiasm involvement with bilateral visual deficits may be caused by direct extension or by traction and distortion of the chiasm. In later stages, optociliary shunt vessels and marked proptosis are detected. Computed tomography (CT) and magnetic resonance imaging (MRI) show typical findings of thickening of the optic nerve, sometimes accompanied by calcification within the tumor. ONSMs appear as contrast-enhancing lesions encasing () or compressing and displacing () the optic nerve. 3-T MRI provides enhanced definition of the nerve (), allowing better resolution of the nerve-tumor spatial relationship and optimization of the radiosurgical treatment planning.
Figure 1. Case II. Axial (a), coronal (b) and sagittal (c) gadolinium-enhanced T1 MR sequences show an atrophic but clearly visible right optic nerve encased by a globular contrast-enhancing homogeneous lesion. The axial cut shows well the perineural growth pattern typical of ONMS, while the entire intraorbital course of the nerve can be appreciated. The same lesion appears on CT imaging [illustrated here in the context of the treatment plan (d)] as a large contrast-enhancing lesion encasing the right optic nerve.
![Figure 1. Case II. Axial (a), coronal (b) and sagittal (c) gadolinium-enhanced T1 MR sequences show an atrophic but clearly visible right optic nerve encased by a globular contrast-enhancing homogeneous lesion. The axial cut shows well the perineural growth pattern typical of ONMS, while the entire intraorbital course of the nerve can be appreciated. The same lesion appears on CT imaging [illustrated here in the context of the treatment plan (d)] as a large contrast-enhancing lesion encasing the right optic nerve.](/cms/asset/abce22b8-235c-4f20-a657-0641b7101d17/icsu_a_622615_f0001_b.gif)
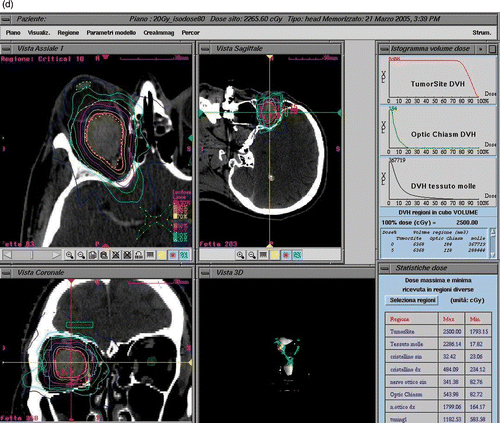
Figure 2. Treatment planning for patient I. CT imaging shows a contrast-enhancing globular lesion growing eccentrically to the left optic nerve, which is compressed and displaced. Treatment planning details such as dose delivered and DVHs are illustrated.
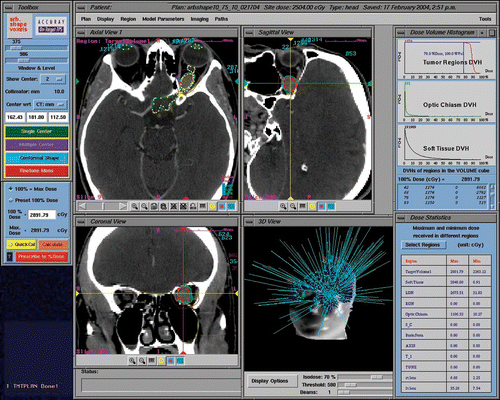
Figure 3. CyberKnife radiosurgery treatment planning for a right ONSM. This patient was a 28-year-old female presenting with progressive deterioration of visual fields and acuity in the right eye over the previous 6 months. (a) Optic nerve imaging was performed using 3-T MR (Siemens Trio). CT-MR fusion was performed on the CyberKnife treatment planning station. The nerve was identified as a loop displaced downward by the tumor and was drawn as a critical structure. This enhanced the degree of nerve sparing from irradiation. (b) A total of 109 beams were delivered non-isocentrically to the lesion. Treatment volume was 0.764 cc. A total dose of 20 Gy prescribed to the 70% isodose was delivered in 4 stages (with 5 Gy per stage) separated by an interval of 24 hours. Maximum dose was 28.57 Gy. Tumor and optic nerve dose volume histograms (DVHs) show a substantial sparing of the optic nerve.
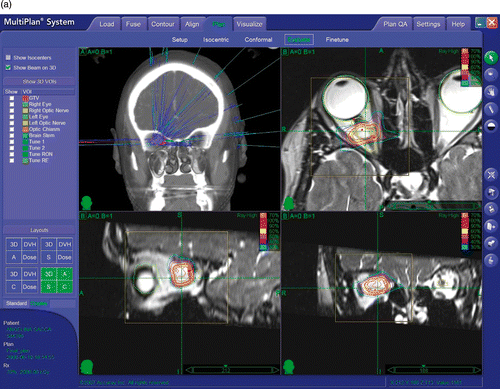
Figure 4. Visual fields before and after treatment for patient I. The upper row corresponds to the left eye, the lower row to the right eye. On each row, the first box is the visual field pre-treatment, the second box is the field after 6 months, and the third box is the field after 1 year. This patient maintained stable visual fields thereafter, with follow-up now approaching 8 years.
Because of their intimate relation to the optic nerve and their sharing of a common pial blood supply, ONSMs present a rather unique treatment challenge. Their typically slow development and the high morbidity of microsurgical resection have encouraged a conservative, watch-and-wait approach. However, progressive deterioration of vision over time has been reported in approximately 85% of patients with prolonged follow-up Citation[1–3], while long-standing visual deficits are less likely to be reversed by treatment. Microsurgical resection is usually reserved for patients with a long history and near-complete or complete loss of vision in the affected eye. Even in very experienced hands, blindness typically follows microsurgical resection of ONSMs Citation[4–6].
Conventional radiotherapy, either as an adjuvant to surgery or alone, has been shown to be moderately effective and reasonably well tolerated; an early review of published studies with a total of 12 patients by Dutton Citation[1] indicated improvements in visual acuity in 75% of patients. Radiation-induced neuropathy and retinopathy, dry-eye syndrome and various adnexal complications have been described, particularly when doses higher than 54 Gy and high fractional doses (1.9 Gy or higher) are used Citation[7].
A more conformal technique developed in the last decade, stereotactic fractionated radiation therapy (SFRT), has been successfully applied to the treatment of ONSMs Citation[8], Citation[9]. Primary SFRT has preserved vision in patients with ONSM better than observation alone. Based on its safety and efficacy, and concerns about injury from single-session SRS, SFRT has been proposed as a standard treatment approach for ONSM. Nevertheless, residual dose spillage over the retina and other nearby structures has occasionally led to loss of vision due to radiation retinopathy or vascular occlusion of retinal vessels. Long-term ophthalmic, adnexal and peripheral complications secondary to conventional irradiation or SFRT have been reported and include iritis, temporal lobe atrophy and endocrine failure Citation[10], Citation[11]. Optic neuritis has been also described following SFRT Citation[12].
Stereotactic radiosurgery involves the precise delivery of a very high dose of radiation with the goal of ablating a small-to-medium-sized intracranial target, with sparing of nearby structures. While SFRT relies on a combination of tissue sparing and daily fractionation to protect the optic nerve, radiosurgery provides extremely tight dose distributions and conformality, restricting the irradiation to the tumor and offering the highest degree of perilesional tissue protection, with an unchallenged 80 to 20% dose fall-off within 3 mm of the target. Frame-based radiosurgery requires drastic measures for the immobilization of the patient and accurate targeting of the lesion, with the entire treatment being typically delivered in a single session, or stage, of 12 to 14 Gy: this dose greatly exceeds the tolerance of the optic nerve Citation[13]. The advent of frameless image-guided radiosurgery in recent years offers the chance to split the radiosurgical dose into several fractions (usually two to five) while keeping the highest degree of conformality and accuracy. The Stanford University group pioneered the novel use of staged radiosurgery for the treatment of optic pathway lesions Citation[14], Citation[15]. Based on this experience, staged radiosurgery has been applied to the treatment of ONSM using a fractionation protocol Citation[16].This study describes five patients with primary ONSM treated using a robotic image guided radiosurgery system and the functional outcome of this treatment based on periodic assessment of visual fields and acuity.
Patients and methods
Patients
Five patients (4 female, 1 male) ranging in age from 28 to 64 years (mean: 47.2 years) with a diagnosis of primary ONSM were treated with staged CyberKnife radiosurgery. Patient and treatment characteristics are summarized in . The first three patients have already been discussed in a previous report Citation[16]; the current paper provides a larger series with a much longer follow-up period. The interval between onset of symptoms and treatment ranged from 6 months to 4 years. Slow worsening of vision characterized all the cases (except for patient V, presenting with sudden deterioration). No detectable growth of the tumors was noticed on serial imaging. All the patients retained a degree of residual vision in the affected eye, while patient IV could only see through the affected eye, having lost vision in the other eye years before due to diabetic retinopathy. Surgery was not offered to these patients to avoid loss of vision in the affected eye (or total blindness in the case of patient IV).
Table I. Patient and lesion characteristics, treatment parameters, outcome and follow-up duration.
Diagnosis of primary ONSM was based on clinical history (visual complaints such as peripheral constriction of visual fields, reduced visual acuity, proptosis or frank exophthalmos) and orbital imaging showing findings typical of ONSM. Open biopsy was not performed. The tumor involved the orbital segment in 4 cases and the canalicular segment in one (patient III).
Each patient was affected by campimetric deficits (bilateral in three, unilateral in two). In the three patients (I, II and IV) with bilateral field deficits, the side affected by the ONSM showed much more pronounced involvement in two cases (I, II), while the other (IV), affected by long-standing type I diabetes and concomitant bilateral retinopathy, came to observation with the eye affected by the ONSM retaining serviceable vision while the other eye was almost blind. Visual acuity was impaired in all patients: patients I and V presented with 20/30 vision while patients II, III and IV presented with 20/40. Two patients (I, IV) also presented also with proptosis and one (III) with severe exophthalmos and optociliary shunt vessels. Radiosurgical treatment was performed within 6 months (I) to 4 years (IV) from the onset of symptoms.
Procedures
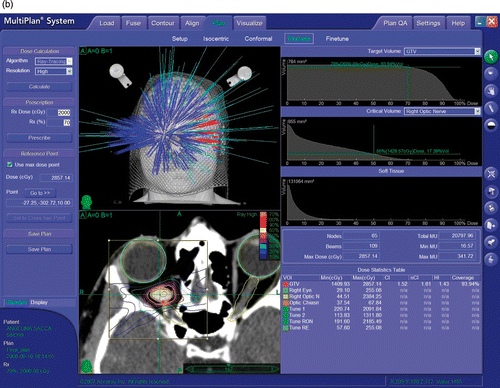
The first three patients underwent a contrast-enhanced, thin-sliced (1.25 mm) CT scan and a volumetric contrast-enhanced 1.5-T MR study (complemented by T1, T2, FLAIR and PD sequences inclusive of axial, coronal and sagittal cuts). The last two patients were studied using 0.5-mm thickness CT and 3-T MR. A 20-Gy total dose was prescribed to the 70% isodose line and delivered in 4 fractions of 5 Gy each. The inverse planning system was instructed to keep the maximum dose below 30 Gy (mean: 27.76 Gy; range: 25–28.9 Gy). The targets were identified on the fused images; however, the contouring of the target and of the critical structures was performed, when possible, on the CT volume, due to its superior spatial reliability related to the absence of magnetic distortion. In all but one case the drawing of the tumor and optic nerve could be easily done using CT imaging alone. In one case (Patient V), 3-T MR added valuable inputs regarding the course of the optic nerve in relation to the tumor. In all cases, the optic nerve was identified (running peripherally around the tumor in four cases and being inglobated within the tumor in one case [patient III]). In those 4 cases where the tumor compressed and displaced the optic nerve, it was possible to delineate and designate the optic nerve as a critical structure during creation of the treatment plan. The optic nerve in these 4 cases remained outside the prescription isodose (70%), being placed within the 50% isodose (patients I and III), the 30% isodose (patient V) or the 20% isodose (patient IV). As a direct consequence of the ability of treatment planning to develop isodose distributions minimizing the optic nerve dose, the total dose and dose/fraction to the optic nerve was kept to an average of 11.2 Gy (range: 8 to 20 Gy) and 2.8 Gy (range: 2 to 5 Gy). In the single case where the optic nerve was encased within the tumor, no attempt was made to draw it and the nerve received the same dose as was given to the tumor. An effort was also made to keep the dose to the retina, lens, and portions of the optic nerve outside the tumor to a minimum.
The treatment was administered using the CyberKnife® Robotic Radiosurgery System (Accuray, Inc., Sunnyvale, CA). Briefly, the CyberKnife is characterized by a 6-MV linear accelerator attached to a robotic manipulator, a non-isocentric treatment planning system, and a target localization mechanism based on real-time X-ray imaging. During the treatment, digitally reconstructed radiographs generated from the planning CT are automatically registered, using the bony landmarks of the skull, with the orthogonal X-ray images obtained frequently during sessions. Based on the target position revealed by the imaging system, the linear accelerator is moved to numerous different positions within the room, emitting 100 to 200 highly collimated beams to reproduce the dose distribution specified in the treatment plan. An average plan took approximately 25 min to deliver. A custom-made plastic mask attached to the treatment couch was used to provide mild restraint during the treatment delivery.
Patients were administered 4 mg dexamethasone at the end of each treatment session. Follow-up included serial MR imaging (every 6 months for 2 years, then once a year) and assessment of visual fields and acuity every 6 months. Imaging and ophthalmological exams were performed and assessed by independent radiologists and ophthalmologists and then reviewed at our institution.
Results
Vision outcomes are listed for each patient in . Median follow-up was 74 months (range: 36 to 88 months). Stable tumor size was detected on serial imaging. A progressive improvement in both visual fields and acuity was documented by an independent ophthalmologist in all but one patient. At the end of the first year post-treatment, a marked visual improvement with near-total restoration of visual fields and acuity was achieved in four of the five patients, and this restoration remained stable over the course of the follow-up period. One patient, also affected by diabetic retinopathy, showed a modest improvement after 6 months, remaining stable thereafter. Two patients (I and V), presenting with bilateral visual impairment due to the distortion of the optic chiasm, experienced bilateral visual improvement at 6-month follow-up, attaining full recovery at 12 months. Proptosis disappeared in one patient and remained stable in the other. The patient with frank exophthalmos harboring the largest meningioma (6.4 cm3) experienced a slight reversal of this condition accompanied by remission of the shunting of the optociliary vessels one year post-treatment.
Discussion
We have shown that in a small cohort of patients with ONSMs fractionated stereotactic radiosurgery could produce excellent tumor control and, in most cases, improvement in visual symptoms. Good clinical outcomes combined with a very low rate of complications would seem to be minimal requirements for the treatment of such lesions. In fact, given the slow growth of these benign lesions, it is worth asking whether they should be treated at all. In 2002 Egan and Lessell published their observations on the natural progression of ONSM Citation[17] and reported that, with a 6.2-year follow-up, approximately 50% of the untreated patients maintained stable visual acuity and three out of 16 patients actually showed a slight improvement over time. These results prompted the researchers to warn the neurosurgery community against treating patients with ONSM with stable conditions and thereby subjecting them to unnecessary risk. A relatively large retrospective study published in 2002 Citation[3] included 64 patients that were either simply observed or treated with surgery only, with radiotherapy only, or with a combination of surgery and radiotherapy. Although the very different patient characteristics and treatment regimens made firm conclusions somewhat difficult, the findings can be summed up as not very promising for any of the groups. The radiation-only group demonstrated the fewest complications and the smallest degree of deterioration of visual acuity. These patients received 40 to 55 Gy conventional or conformal radiotherapy, and at a mean 150.2-month follow-up they experienced a 33.3% complication rate (including retinopathy, iritis and temporal lobe atrophy), but did not show a statistically significant drop in acuity. Certainly, if surgical or radiation-based treatment is pursued, the potential benefit should be measured against the risk of causing or hastening blindness in relatively stable patients.
Aside from a few isolated case studies, the first documented relatively conformal radiation treatment of ONSM was conducted by a group from the University of California San Francisco in 1992 and involved four patients treated with an immobilization device that aligned the optic nerve with a vertical axis and used three small half-beams blocked along this axis, thus shielding the optic nerve Citation[18]. The technique irradiated a relatively small (58 cm3) volume with the dose to the 80% isodose line. However, this group did not report on the outcome of the treatment.
Pitz et al. Citation[9] were the first to treat primary ONSM with a fully fractionated course of stereotactically targeted radiation, an approach known as fractionated stereotactic radiation therapy (FSRT). They treated 15 patients with 54 Gy; at a mean follow-up of 37 months (range: 12–71 months), visual acuity had improved in one patient, visual fields had improved in six patients, and the other patients remained stable. No one experienced a significant side effect. Secondary ONSM patients faired slightly less well, according to a subsequent report Citation[19]: Six of 24 examined eyes showed improvements in visual acuity, and seven of 26 examined eyes showed improvements in visual fields; 17 and 19 eyes, respectively, maintained stability in visual acuity and visual field.
In the following year, Narayan et al. from the University of Michigan treated 14 patients using the technique of three-dimensional conformal radiotherapy (3D-CRT) and obtained similar results to those obtained using FSRT Citation[20]. Five patients had improvement in visual acuity, two worsened, and the rest remained stable over a median of 51.3 months. Nine patients showed improved visual fields. Baumert et al. from Switzerland treated 23 patients with tumor progression using FSRT with 50.4 Gy delivered over six weeks Citation[21]. With a median follow-up of 20 months, 16 of their patients experienced improvement in vision, five had stable vision, and one got worse. Ninety-five percent of patients achieved stable disease.
More recent studies of FSRT have continued to demonstrate its potential for controlling the growth of ONSMs while stabilizing or reversing visual deficits caused by them. Milker-Zabel et al. Citation[22] treated 15 patients with FSRT as primary treatment (other patients were treated after biopsy or surgery or for recurrences) to a median a median total dose of 54.9 Gy. At a median follow-up of 4.5 years, local tumor control was 100% and 97% of patients showed stable or improved visual acuity. Arvold et al. Citation[23] treated 25 patients with FSRT using photons or protons to a median dose of 50.4 Gy equivalents. At a median follow-up of 30 months, 95% of patients had improved (14 patients) or stable (7 patients) visual acuity, and 95% showed no progression on imaging. A single recurrence was noted 11 years after treatment. Comparable outcomes were reported by Lesser et al. Citation[24]. Thus, FSRT may be gaining acceptance as a low-risk approach to controlling ONSM growth and symptom progression. In fact, Smee et al. Citation[25] reserved stereotactic radiosurgery (see below) for patients whose vision was not likely to be affected by treatment; when preservation or improvement of vision was a therapeutic goal they opted for FSRT. They ultimately obtained 100% tumor control and vision preservation in all but one patient using their methods.
Single-session SRS treatment of ONSMs has also been reported Citation[26]. Sitathanee et al. Citation[27] treated a patient who had already lost vision with a single 15-Gy dose. There were no complications to the contralateral eye.
Three patients were treated with single-session SRS in the report by Smee et al. Citation[25]. As noted above, they recommended SRS only when preservation of vision was not a goal of treatment. Liu et al. Citation[28] reported on outcomes of Gamma Knife SRS to a mean dose of 13.3 Gy for 30 patients. In nine patients who still had vision and whose ONSMs surrounded the optic nerve, SRS was delivered in two sessions. At a median follow-up of 56 months the tumor control rate was 93.3%. Vision deteriorated in 20% of patients; it was not indicated if these patients were treated in a single fraction or in two fractions.
Staged radiosurgery outcome for the treatment of ONSM was preliminarily reported by our group in 2007 Citation[16]. The current paper provides a larger series with prolonged follow-up. Staged radiosurgery was performed with the aim of stopping disease progression and maintaining visual function. Restoration to normal vision in 4 out of 5 patients within 6 to 12 months following treatment was an unexpected gift, most likely related to improved vascular supply to the involved optic nerve following tumor irradiation. The ability to use very tight dose distributions combined with hypofractionation was perhaps associated with the highest degree of protection of the optic nerve that technology has yet made available. In two cases (where a CyberKnife G3 model was used) the optic nerve was included in the 50% isodose, while in the other two cases (using a CyberKnife G4) it was kept outside the 30% isodose. In the first two cases the optic nerve received approximately 2.5 Gy per fraction (for a total dose of 10 Gy in 4 fractions), while in the latter two cases the optic nerve dose per fraction was less than 1.5 Gy (total dose 6 Gy). The tumor received 5 Gy per fraction (total dose 20 Gy). The patient whose optic nerve was enveloped by the ONSM could not be spared from receiving a similar dose to the nerve and tumor. Nevertheless, this patient showed substantial improvement in visual fields and acuity and maintained this improvement over time (the first patient of this series having had a follow-up of longer than 7 years). This small clinical series demonstrates a promising non-invasive approach to treating ONSM. Patients can be treated in less than a week as opposed to the full month required by conventional radiation techniques, including SFRT. The degree of sparing of the optic nerve when this is peripherally displaced by the tumor is unmatched: robotic image guided radiosurgery can provide doses effective at halting the tumor progression and restoring visual function, while at the same time the dose received by the optic nerve itself can be less than 1.5 Gy per fraction (as described for the last two cases treated using a Cyberknife G4).
Conclusions
Robotic image guided radiosurgery proved to be safe and effective in a small series of patients harboring ONSM. These preliminary results pave the way for a prospective multicentric study aimed at investigating the safety and efficacy of staged CyberKnife radiosurgery as a primary treatment option for ONSM.
Declaration of interest: The authors report no conflicts of interest
References
- Dutton JJ. Optic nerve sheath meningiomas. Surv Ophthalmol 1992; 37(3)167–183
- Kennerdell JS, Maroon JC, Malton M, Warren FA. The management of optic nerve sheath meningiomas. Am J Ophthalmol 1988; 106(4)450–457
- Turbin RE, Thompson CR, Kennerdell JS, Cockerham KP, Kupersmith MJ. A long-term visual outcome comparison in patients with optic nerve sheath meningioma managed with observation, surgery, radiotherapy, or surgery and radiotherapy. Ophthalmology 2002; 109(5)890–899, discussion 899–900
- Cristante L. Surgical treatment of meningiomas of the orbit and optic canal: A retrospective study with particular attention to the visual outcome. Acta Neurochir (Wien) 1994; 126(1)27–32
- Delfini R, Missori P, Tarantino R, Ciapetta P, Cantore G. Primary benign tumors of the orbital cavity: Comparative data in a series of patients with optic nerve glioma, sheath meningioma, or neurinoma. Surg Neurol 1996; 45(2)147–153; discussion 153–154
- Mark LE, Kennerdell JS, Maroon JC, Rosenbaum AE, Heinz R, Johnson BL. Microsurgical removal of a primary intraorbital meningioma. Am J Ophthalmol 1978; 86(5)704–709
- Goldsmith BJ, Rosenthal SA, Wara WM, Larson DA. Optic neuropathy after irradiation of meningioma. Radiology 1992; 185(1)71–76
- Jeremic B, Pitz S. Primary optic nerve sheath meningioma: Stereotactic fractionated radiation therapy as an emerging treatment of choice. Cancer 2007; 110(4)714–722
- Pitz S, Becker G, Schiefer U, Wilhelm H, Jeremic B, Bamberg M, Zrenner E. Stereotactic fractionated irradiation of optic nerve sheath meningioma: A new treatment alternative. Br J Ophthalmol 2002; 86(11)1265–1268
- Parsons JT, Bova FJ, Fitzgerald CR, Mendenhall WM, Million RR. Radiation optic neuropathy after megavoltage external-beam irradiation: Analysis of time-dose factors. Int J Radiat Oncol Biol Phys 1994; 30(4)755–763
- Parsons JT, Bova FJ, Fitzgerald CR, Mendenhall WM, Million RR. Severe dry-eye syndrome following external beam irradiation. Int J Radiat Oncol Biol Phys 1994; 30(4)775–780
- Andrews DW, Faroozan R, Yang BP, Hudes RS, Werner-Wasik M, Kim SM, Sergott RC, Savino PJ, Shields J, Shields C, et al. Fractionated stereotactic radiotherapy for the treatment of optic nerve sheath meningiomas: Preliminary observations of 33 optic nerves in 30 patients with historical comparison to observation with or without prior surgery. Neurosurgery 2002; 51(4)890–902, discussion 903–904
- Girkin CA, Comey CH, Lunsford LD, Goodman ML, Kline LB. Radiation optic neuropathy after stereotactic radiosurgery. Ophthalmology 1997; 104(10)1634–1643
- Adler JR, Jr, Gibbs IC, Puataweepong P, Chang SD. Visual field preservation after multisession cyberknife radiosurgery for perioptic lesions. Neurosurgery 2006; 59(2)244–254, discussion 244–254
- Pham CJ, Chang SD, Gibbs IC, Jones P, Heilbrun MP, Adler JR, Jr. Preliminary visual field preservation after staged CyberKnife radiosurgery for perioptic lesions. Neurosurgery 2004; 54(4)799–810, discussion 810–812
- Romanelli P, Wowra B, Muacevic A. Multisession CyberKnife radiosurgery for optic nerve sheath meningiomas. Neurosurg Focus 2007; 23(6)E11
- Egan RA, Lessell S. A contribution to the natural history of optic nerve sheath meningiomas. Arch Ophthalmol 2002; 120(11)1505–1508
- Lindblom B, Truwit CL, Hoyt WF. Optic nerve sheath meningioma. Definition of intraorbital, intracanalicular, and intracranial components with magnetic resonance imaging. Ophthalmology 1992; 99(4)560–566
- Becker G, Jeremic B, Pitz S, Buchgeister M, Wilhelm H, Schiefer U, Paulsen F, Zrenner E, Bamberg M. Stereotactic fractionated radiotherapy in patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys 2002; 54(5)1422–1429
- Narayan S, Cornblath WT, Sandler HM, Elner V, Hayman JA. Preliminary visual outcomes after three-dimensional conformal radiation therapy for optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys 2003; 56(2)537–543
- Baumert BG, Villa S, Studer G, Mirimanoff RO, Davis JB, Landau K, Ducrey N, Arruga J, Lambin P, Pica A. Early improvements in vision after fractionated stereotactic radiotherapy for primary optic nerve sheath meningioma. Radiother Oncol 2004; 72(2)169–174
- Milker-Zabel S, Huber P, Schlegel W, Debus J, Zabel-du Bois A. Fractionated stereotactic radiation therapy in the management of primary optic nerve sheath meningiomas. J Neurooncol 2009; 94(3)419–424
- Arvold ND, Lessell S, Bussiere M, Beaudette K, Rizzo JF, Loeffler JS, Shih HA. Visual outcome and tumor control after conformal radiotherapy for patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys 2009; 75(4)1166–1172
- Lesser RL, Knisely JP, Wang SL, Yu JB, Kupersmith MJ. Long-term response to fractionated radiotherapy of presumed optic nerve sheath meningioma. Br J Ophthalmol 2010; 94(5)559–563
- Smee RI, Schneider M, Williams JR. Optic nerve sheath meningiomas – non-surgical treatment. Clin Oncol (R Coll Radiol) 2009; 21(1)8–13
- Kwon Y, Bae JS, Kim JM, Lee DH, Kim SY, Ahn JS, Kim JH, Kim CJ, Kwun BD, Lee JK. Visual changes after gamma knife surgery for optic nerve tumors. Report of three cases. J Neurosurg 2005; 102(Suppl)143–146
- Sitathanee C, Dhanachai M, Poonyathalang A, Tuntiyatorn L, Theerapancharoen V. Stereotactic radiation therapy for optic nerve sheath meningioma; an experience at Ramathibodi Hospital. J Med Assoc Thai 2006; 89(10)1665–1669
- Liu D, Xu D, Zhang Z, Zhang Y, Li Y, Liu X, Jia Q, Zheng L, Song G. Long-term results of Gamma Knife surgery for optic nerve sheath meningioma. J Neurosurg 1010; 113(Suppl)28–33