Abstract
Introduction: For successful cochlear implantation in difficult ears, image guided navigation systems can help identify surgical landmarks or confirm the surgeon's anatomical knowledge. In this pilot case study, exact navigation based on intraoperative CT scanning was investigated and helped confirm important and necessary landmarks, such as the facial nerve, cochlea and intracochlear structures, and at least adequate placement of a straight electrode array.
Material and Methods: Intraoperative imaging was performed on a 40-slice sliding-gantry CT scanner (Siemens SOMATOM Sensation 40 Open) with an expanded gantry bore (82 cm). Raw image data were reconstructed with a slice thickness and increment of 0.6 mm and were imported to a frameless infrared-based navigation station (BrainLAB VectorVision Sky). In a preoperative accuracy and feasibility study, a phantom skull was scanned and registered five times by the navigation system. Based on the encouraging results, the system was then applied to a male patient with post-traumatic sensorineural hearing loss. The intraoperative target positioning error was measured by a “blinded” colleague who defined the distance of the pointer from different sections of the facial nerve without seeing the intraoperative field.
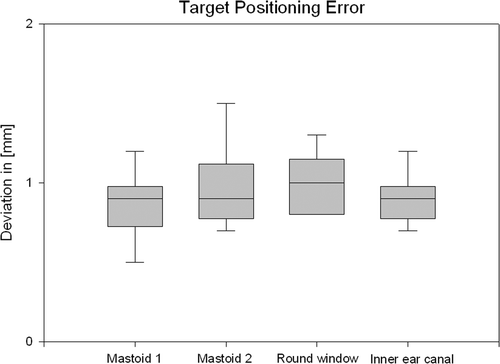
Results: The average deviation in the phantom skull was 0.91 mm (SD 0.27 mm) on the mastoid, 1.01 mm (SD 0.21 mm) on the round window, and 0.9 mm (SD 0.18 mm) on the inner ear canal.
Surgery could be performed without major complications. The distance of the pointer from the facial nerve could be defined exactly using navigation in ten measurements. The cochleostomy and electrode insertion were performed with the aid of navigation. After insertion, direct intraoperative control of the electrode position was achieved by means of a low-dose CT scan. Two months postoperatively, the patient had a satisfactory open-set speech understanding of 85%.
Conclusion: With the use of intraoperative acquisition of CT images (or digital volume tomography devices) and automatic volumetric registration for navigation, surgical precision can be improved, thereby allowing successful cochlear implant surgery in patients with complex malformations or who have undergone multiple previous ear surgeries and consequently lack anatomical landmarks. Our study clearly shows that this high-technology combination is superior to other registration methods in terms of accuracy and precision. Further investigations should aim at developing automatic segmentation and applications for minimally invasive surgery of the lateral skull base.
Introduction
Successful cochlear implantation in normal and, especially, malformed inner- and middle ears requires precise localization of anatomical landmarks (i.e., the facial nerve, ossicles, cochlea and vestibular organs) and insertion of the array and verification of its position. As the indications for cochlear implant surgery expand, complex malformations are no longer a contra-indication. In these situations, image guided navigation systems, which can help identify surgical landmarks or confirm the surgeon's anatomical knowledge intraoperatively, are of particular interest. Implantation in ears with malformations or following trauma is known to be challenging, and may involve a higher risk of incorrect placement of electrode arrays, facial nerve damage, or other complications Citation[1]. Image guided navigation can prevent misplacement of electrodes Citation[2] and is required for the further development of percutaneous cochlear implantation Citation[3].
For precise navigation, markers must be attached to the patient's body prior to image data acquisition. In the registration phase, the image data is matched to the patient intraoperatively by locating the fiducial markers on the patient's body. The safest method for achieving a reliable registration is by fixation of fiducial markers in the patient's bone with screws, as this prevents the displacement which can occur with soft tissue-mounted fiducials Citation[4] or dental reference arrays Citation[5]. In fact, the use of titanium screws drilled into the skull is considered the gold-standard method Citation[6]. Recently, intraoperative high-resolution computed tomographic (CT) imaging combined with image guided navigation has become available, which might enable improved intraoperative localization of anatomic structures and the electrode array Citation[7]. This technology has potential to save time and resources, and possibly avoid the revision surgery associated with improper electrode positioning Citation[2].
We developed a concept, described in this pilot case study, wherein precise navigation based on intraoperative CT scanning helped confirm important and necessary landmarks, such as the facial nerve, cochlea, and intracochlear location, and at least adequate placement of a straight electrode array. The male patient had undergone previous ear surgeries, specifically facial nerve decompression because of bilateral facial palsy after temporal bone fractures, and was scheduled for a cochlear implant because of progressive sensorineural hearing loss. He agreed to have his preoperative CT scan performed under general anesthesia in the operating room. Intraoperative computed tomography (iCT) was used as the basis for data acquisition, as well as for direct intraoperative control of the insertion result.
Materials and methods
Preoperative accuracy tests
An alternative to fiducial marker placement in head surgery is the use of an intraoperative CT (iCT) with automatic image registration. With this approach, invasive fixation using bone screws becomes unnecessary. The short acquisition times and high spatial resolution associated with this technique enable integration of intraoperative navigation with automatic image registration after scanning. The concept has already proven its accuracy for neurosurgical and orthopaedic interventions Citation[8]. The aim of the preoperative study was to evaluate the accuracy of the automatic registration using iCT in phantom-based targeting trials. Based on the encouraging laboratory results, it was then applied to a patient.
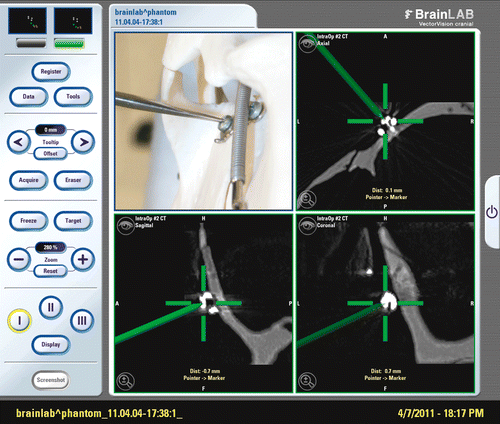
The phantom and patient were placed in the final prone surgical position after correction of misalignment by positioning them on an adjustable, flexible and rotatable radiolucent operating table (JUPITER OR table, Trumpf, Puchheim, Germany). The JUPITER table permits movement to a previously stored scanning position and then back to the operating position at any time during surgery. A carbon head plate (OMI, Inc., Cincinnati, OH) was used as a headrest. During image acquisition the gantry moves over the patient, with the position of the catheters and ventilation tube remaining stable during the scanning procedure. Intraoperative imaging was performed on a 40-slice sliding-gantry CT scanner (SOMATOM Sensation 40 Open, Siemens Healthcare, Forchheim, Germany) with an expanded gantry bore (82 cm) and 40 × 0.6 mm collimation. Raw image data were reconstructed with a slice thickness and increment of 0.6 mm using a dedicated skull base reconstruction kernel in axial orientation. This resulted in a voxel size of 0.4 mm × 0.4 mm × 0.6 mm in the field of view. The measured dose length product (DLP) was 139 mGy*cm, the CTDI volume was 55.6 mGy, and the effective dose was 0.3 mSv. Data were imported to a frameless infrared-based navigation station (VectorVision Sky with Cranial Software 7.9, BrainLAB, Feldkirchen, Germany). The volumetric dataset was registered automatically by the navigation system (which was linked to the scanner) by means of the 3D reference structure of the skull reference array (shown in ). Accuracy was then measured as the deviation of the real pointer position in situ from the pointer position indicated by the navigation system (Target Positioning Error [TPE]) (). The deviation was measured in millimeters in all three orientations, i.e., coronal, sagittal and axial, and the value of the maximum deviation was determined. To achieve valid and exact results, corresponding target points had to be defined:
Two titanium screws were fixed on the mastoidal bone beside the outer ear canal.
One titanium screw was fixed on the round window.
One titanium screw was fixed on the inner ear canal
Figure 1. Phantom skull with skull reference array on the temporal bone in the iCT. To avoid metallic artifacts, the reference star must be outside the scanning area.
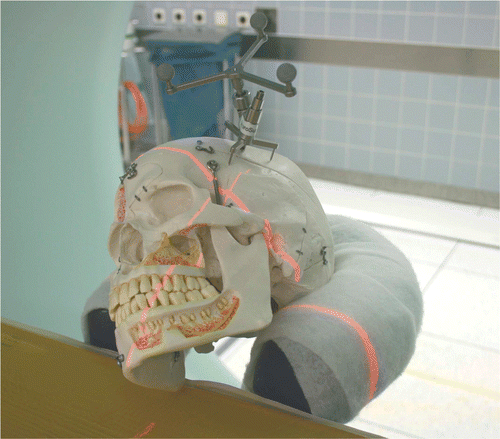
The phantom was registered five times by the navigation system. For each registration, the navigation system and iCT were re-booted, the skull reference array was replaced on the phantom, and deviation of all target markers was measured again.
Patient history
A 44-year-old male patient presented with post-traumatic hearing loss in the form of total deafness on the left side and residual hearing on the right side subsequent to a car accident in 1992. The patient's left facial nerve had to be decompressed following the initial trauma because of a total palsy due to multiple petrosal fractures. The patient presented preoperatively with a left facial palsy of grade II on the House-Brackmann scale. Two years after facial rehabilitation in 1994, a tympanoplasty was performed to restore the ear drum. However, in the last two years, the patient had complained about progressive hearing loss and otalgia on the left side.
Preoperatively, the transplanted ear drum appeared intact, with no signs of infection or cholesteatoma. The MRI showed liquid-like structures in the left mastoid.
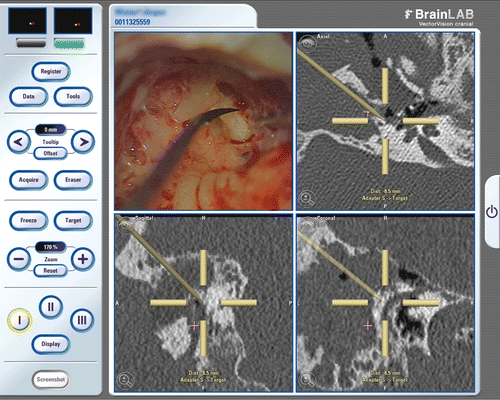
After the patient had given informed consent to the surgery and agreed to the iCT examinations, an intraoperative CT scan was performed after sterile fixation of the skull reference array on the temporal bone with a single titanium screw (). The iCT dataset was then transferred and automatically registered to the navigation system.
Results
Figure 3. The sterile-draped patient with the skull reference array fixed in place undergoing the intraoperative CT scan.

The skull reference array could easily be attached to the phantom and to the patient using a single self-cutting titanium screw. No additional drill hole was required. The average deviation over five measurements was 0.91 mm (SD 0.27 mm) on the mastoid, 1.01 mm (SD 0.21 mm) on the round window, and 0.9 mm (SD 0.18 mm) on the inner ear canal ().
Another important parameter is the additional time required for navigation and set-up. Although the motivation to engage in this approach is primarily dependent on a positive outcome of the surgery, the additional time/effort has to be acceptable. For the phantom skull studies, the following time periods were measured after an initial learning curve of four registrations:
System boot-up with self-test (navigation + iCT): 12 min.
Implementation of skull reference array: 7 min.
Intraoperative CT scan (including scout and reconstructions): 3 min.
Data transfer, automatic registration, and verification: 1 min.
Total duration: 23 min.
First clinical application
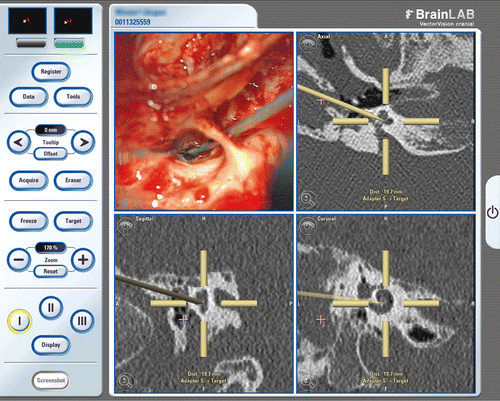
Surgery was performed without major complications. Re-opening the mastoidectomy revealed a cholesteatoma, which had to be removed completely from the cavity and the exposed facial nerve. To determine the exact target positioning error (TPE), corresponding anatomical landmarks are crucial. Hence, the facial nerve had to be clearly identified, and served well as a target marker. The intraoperative TPE was then measured by a “blinded” colleague who defined the distance from the pointer to different sections of the facial nerve without seeing the intraoperative field (). In ten cases the distance to the facial nerve could be defined exactly with the aid of navigation. By these means, the cochleostomy and electrode insertion were performed with the aid of navigation (). After insertion, direct intraoperative control of the electrode position was accomplished by a single-shot CT scan (scout + 2 slices at 1 mm reconstruction) to decrease the X-ray exposure. Two months postoperatively, the patient has an open-set speech understanding, scoring 85% on the Freiburg numbers test, 35% on the Freiburg monosyllables test, and 32% on the Oldenburg sentence test at 70 dB SPL. No facial nerve stimulation was observed during surgery, and no complications were encountered.
The additional time periods required during the initial clinical application of the iCT for this procedure on the live patient were much longer than the corresponding durations for the phantom study. The times for the patient were as follows:
System boot-up with self-test (navigation + iCT): 12 min.
Implementation of skull reference array: 35 min.
Sterile covering of CT gantry: 25 min.
Intraoperative CT scan (with scout and reconstructions): 25 min.
Data transfer, automatic registration, and verification: 1 min.
Instrument calibration (curved and straight needles): 1 min.
Total duration: 113 min.
Discussion
The use of surgical navigation is well accepted in anterior skull base and sinus surgery Citation[9], but not in surgery of the lateral skull base because of the reduced accuracy associated with prolonged surgical duration for referencing and registration Citation[5]. Navigation data are usually based on preoperative CT or MR images Citation[10]. With the intraoperative acquisition of CT images to serve as a basis for navigation, surgical precision can be improved, thereby allowing successful cochlear implant surgery in patients with complex malformations or those who have undergone multiple previous ear surgeries and consequently lack anatomical landmarks. Our study clearly shows that the high-resolution dataset of iCT with automatic volumetric registration is superior to other registration methods (i.e., surface matching Citation[11], Citation[12], point-pair matching Citation[5], Citation[13], and even pin registration Citation[14]), not only in terms of accuracy and precision, but also in time and effort required. In volumetric registration the highest spatial accuracy is obtained in the center of the operation field, which is normally the region of interest, whereas in all other registration methods the best accuracy is obtained near the registered region (in surface matching on the surface; in pin registration between the pins; and in point-pair matching between the points), and progressively decreases as the surgeon moves away from that region. This is a severe problem, especially in cochlear implantation, because the insertion point of the cochlear implant is deep in the mastoid and not on the surface or in the region of pin registration.
As the set-up for navigation and data acquisition was performed immediately prior to surgery, there was no time delay between the navigated controlled mastoidectomy and the cochleostomy. Direct intraoperative control with CT allows precise evaluation of the electrode position and reduces the risk of additional procedures being required or the need for subsequent CT evaluation outside the OR. Modern iCT scanners and intraoperative digital volume tomography (iDVT) devices could be combined with navigation systems and could offer sufficiently high resolution to separate the vestibular scale from the tympanic scale for correct insertion of the electrode Citation[15], Citation[16]. According to the literature, it remains unclear whether iCT or iDVT is superior with regard to the resolution and artifacts in cochlear implantation Citation[15], Citation[17]. However, the combination of navigation based on intraoperative data and direct radiological control can improve surgical quality [7] and reduce total surgical time and the risk of complications, and is therefore a valuable tool in the performance of cochlear implant surgery and general ear surgery in traumatic operating fields or in cases of revision cholesteatoma, malignant tumor, or paraganglioma.
Because of the high acquisition costs of such devices (approximately 240,000 Euros), the usage rate needs to be as high as possible to achieve an acceptable level of cost effectiveness. Therefore, interdisciplinary, versatile use by neurosurgeons, maxillofacial surgeons and orthopaedic surgeons is required. The ability to scan all areas of the body makes the system useful for numerous surgical subspecialties. Positioning of the patients remains as usual, and the surgical workflow is basically unchanged. There is no need for special surgical instruments or dedicated anesthesiological devices. The scanning position of the table is digitally stored after a preoperative safety check to enable intraoperative acquisition of control CTs without danger of collision between the gantry and the patient or table, even under impaired visual control due to a draped patient and table. Using this concept, there was no case of collision or tube dislocation due to the scanning procedure in a series of 94 neurosurgical cases Citation[8]. A large working space between the mobile CT gantry and the operating table allows the regular use of an operating microscope. Electrophysiological monitoring was used and is not affected by the scanner; nor does it interfere with image acquisition.
In this pilot study, navigation was used for location and verification of anatomical landmarks, i.e., the facial nerve (anterior, posterior), cochlea, and the ascending part of the cochlear lumen. It could be shown that it is possible to use navigation with a high degree of accuracy, registered by iCT to confirm landmarks on the lateral skull base intraoperatively. Submillimetric accuracy is not always mandatory for clinical identification of landmarks, but is required in minimally invasive ear surgery like percutaneous cochlear implantation. Hence, such navigation systems could be promising additional tools, even for cases involving malformations and complex surgical situations. Further investigations with this new technology should aim at developing automatic segmentation and applications to minimally invasive surgery of the lateral skull base. For this purpose, and to determine the exact workflow of iCT and navigation, a prospective study with a larger number of cases is planned.
Declaration of interest: The authors report no declaration of interest.
References
- Tucci DL, Telian SA, Zimmerman-Phillips S, Zwolan TA, Kileny PR. Cochlear implantation in patients with cochlear malformations. Arch Otolaryngol Head Neck Surg 1995; 121(8)833–838
- Bloom JD, Rizzi MD, Germiller JA. Real-time intraoperative computed tomography to assist cochlear implant placement in the malformed inner ear. Otol Neurotol 2009; 30(1)23–26
- Labadie RF, Balachandran R, Mitchell J, Noble JH, Majdani O, Haynes D, Bennett M, Dawant BM, Fitzpatrick JM. Clinical validation study of percutaneous cochlear access using patient-customized microstereotactic frames. Otol Neurotol 2010; 31(1)94–99
- Eggers G, Mühling J, Marmulla R. Image-to-patient registration techniques in head surgery. Int J Oral Maxillofac Surg 2006; 35(12)1081–1095
- Stelter K, Ledderose GJ, Tschiesner U, Matthias C, Spiegl KE. Clinical application of a new dental reference system for computer assisted surgery at the lateral skull base. The Open Otorhinolaryngology Journal 2008; 2(2)49–56
- Eggers G, Mühling J, Marmulla R. Template-based registration for image-guided maxillofacial surgery. J Oral Maxillofac Surg 2005; 63(9)1330–1336
- Schipper J, Klenzner T, Aschendorff A, Arapakis I, Ridder GJ, Laszig R. [Navigation-controlled cochleostomy. Is an improvement in the quality of results for cochlear implant surgery possible?] [In German]. HNO 2004; 52(4)329–335
- Tonn JC, Schichor C, Schnell O, Zausinger S, Uhl E, Morhard D, Reiser M. Intraoperative computed tomography. Acta Neurochir Suppl 2011; 109: 163–167
- Stelter K, Andratschke M, Leunig A, Hagedorn H. Computer-assisted surgery of the paranasal sinuses: Technical and clinical experience with 368 patients, using the Vector Vision Compact system. J Laryngol Otol 2006; 120(12)1026–1032
- Aschendorff A, Maier W, Jaekel K, Wesarg T, Arndt S, Laszig R, Voss P, Metzger M, Schulze D. Radiologically assisted navigation in cochlear implantation for X-linked deafness malformation. Cochlear Implants Int 2009; 10(Suppl 1)14–18
- Ledderose GJ, Stelter K, Leunig A, Hagedorn H. Surface laser registration in ENT-surgery: Accuracy in the paranasal sinuses – a cadaveric study. Rhinology 2007; 45(4)281–285
- Marmulla R, Eggers G, Mühling J. Laser surface registration for lateral skull base surgery. Minim Invasive Neurosurg 2005; 48(3)181–185
- Marmulla R, Mühling J, Eggers G, Hassfeld S. [Markerless patient registration. A new technique for image-guided surgery of the lateral base of the skull] [In German]. HNO 2005; 53(2)148–154
- Grauvogel TD, Soteriou E, Metzger MC, Berlis A, Maier W. Influence of different registration modalities on navigation accuracy in ear, nose, and throat surgery depending on the surgical field. Laryngoscope 2010; 120(5)881–888
- Aschendorff A, Kromeier J, Klenzner T, Laszig R. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear 2007; 28(2 Suppl)75S–79S
- Fama A, Carlson M, Driscoll C, Lane J. The use of micro-CT to evaluate cochlear implant electrode position and intracochlear damage. Laryngoscope 2010; 120(Suppl 4)S205
- Kyriakou Y, Kolditz D, Langner O, Krause J, Kalender W. [Digital volume tomography (DVT) and multislice spiral CT (MSCT): An objective examination of dose and image quality] [In German]. Rofo 2011; 183(2)144–153