Abstract
Three hundred and eighty computer-assisted total knee arthroplasty cases were reviewed for accuracy of mechanical alignment. The 331 patients in the first set, designated Group A, showed a consistent valgus error of 1° from neutral alignment. It was hypothesized that a manual 1° varus correction during femur resection would yield a significantly greater level of accuracy in the second set of 49 patients, designated Group B. A mechanical alignment of ±3° was achieved in 91% of the uncalibrated Group A patients, which was significantly lower (p = 0.035) than the rate of 98% achieved in the calibrated Group B. Further statistical analysis of the data showed the time expenditure was not significantly changed once a new target value was recalibrated. By quantifying mean errors of measures at an early timeframe, enhanced accuracy in CAS can be achieved.
Introduction
Balancing the risks and benefits of different approaches to total knee replacement (TKR) has been widely debated. The most controversy surrounds minimally invasive and computer-assisted surgery (CAS) approaches to TKR. Previous studies have indicated that minimally invasive total knee replacement surgery (MIS-TKR) leads to shorter hospital stays and early recovery enhancement Citation[1–3]. Conversely, research has also shown that MIS-TKR leads to higher rates of revision and other complications, such as prolongation of the procedure Citation[4]. MIS-TKR, while potentially beneficial in improving recovery time, has recently been criticized for leading to an increased rate of component malpositioning and early failure Citation[5]. The procedures in this study were each performed via a minimally invasive approach in combination with computer navigation.
In traditional exposure, conventional instrumentation has performed reasonably well in providing accurate alignment Citation[3]. The adoption of CAS to guide the surgeon in achieving optimal positioning and alignment in MIS has not been universally accepted for a number of reasons: The technology is more costly, more time-consuming, and requires additional effort on the part of the surgeon and other healthcare personnel Citation[6–11]. With conventional surgical incision, the use of CAS has been shown to reduce the prevalence of component misalignment (±3°) from 20% to 5% Citation[8–12], Citation[18]. While only limited information is available regarding the outcomes associated with MIS-TKR, even less is known about the accuracy of individual components in this setting Citation[3], Citation[13].
In the present study, electromagnetic computer-assisted surgery (EM-CAS) was used via a minimally invasive approach. The study was designed to look at accuracy in a single surgeon series, with the emphasis on means by which surgeons might achieve an even greater level of accuracy than previously reported by correcting for any consistent errors detected. No attempt was made to discern whether the error was surgeon- or CAS-induced; rather, it was planned to normalize all the errors as one and use the increased precision achieved with CAS to enable future recalibration of the CAS system once an error was detected.
The study was undertaken with three fundamental goals. The first was to determine the level of accuracy that can be achieved by one surgeon using one particular CAS system; the second was to determine, after conducting a midterm assessment, the improvement in accuracy that can be achieved in a second patient cohort with manual surgeon adjustments derived from the mechanical alignments achieved in the first group. Finally, we also assessed the presence or absence of any merits of MIS-CAS with respect to procedure time and the amount of additional time (if any) related to this recalibration process.
Materials and methods
Three hundred and eighty patients undergoing total knee replacement (TKR) by one surgeon (D.R.L.) at Methodist Hospital System in Houston, TX, were enrolled in a prospective study over a 2.5-year period from 24 January 2006 through 2 June 2007; each patient was followed for a minimum of two years after the procedure. The study protocol received full approval from the Institutional Review Board. The 380 patients were comprised of 258 females with an average age of 69 years, and 122 males with an average age of 68 years. Of the entire study sample, 95% had osteoarthritis and 5% had rheumatoid arthritis, with 9% of the whole population being diabetic. The inclusion criterion for all patients was radiographic evidence of degenerative or inflammatory arthritis requiring total knee replacement. There was no exclusion for age, deformity, presence of metal hardware, or previous surgical history. A suggested body mass index of less than 35 kg/m2 was strongly encouraged. No other family or social histories were excluded in order to replicate a real-world cross-section of a typical practice patient population.
The operative technique was similar to that described by Tria and Coon Citation[2]. All procedures were performed with the same prosthesis design (NexGen knee implant, Zimmer, Warsaw, IN), with either cruciate-sparing or substituting designs being selected depending on demand and ligament integrity at the time of surgery. Measurements from the CAS procedure were made using a Medtronic AxiEM System (Medtronic Navigation, Inc., Louisville, CO). During the study period, no modifications were made to the CAS system for baseline non-calibrated (Group A) or recalibrated (Group B) in terms of software, upgrades, or field generators.
During the surgery each bone resection was archived in the CAS software and recorded for comparisons of individual measurement accuracy against standardized postoperative 36-inch anterior posterior (AP) and lateral radiographs. Each intraoperative CAS component measurement was therefore discretely compared to its resultant X-ray angle to minimize additive errors, as well as to prevent cancellation of errors which may occur in overall measurements of alignment. Standardized positioning X-ray techniques were used to minimize parallax errors arising from variations in the projection rotation of extremities. All radiographic measurements were made by independent blinded researchers using a trigonometric calculation, as opposed to a goniometer measurement using mid-axes of the bony canal. Postoperative 36-inch X-rays were obtained in all patients and used to assess the mechanical alignment. Using the Knee Society angle assignment convention, the alpha, beta, gamma and delta angular positions of each component in the AP and sagittal planes were recorded, as well as the joint line position, using the modified Insall-Salvati ratio for changes in translation with anatomic measurements Citation[1].
After completing the assessment of 331 patients (Group A), we found that the mean and standard deviation of absolute error for patients in this group were 2.06 and 1.31, respectively. A power analysis was performed to determine the optimal number of patients to assess angular trending errors on the part of the surgeon/CAS team. To determine how many procedures would have to be performed in a theoretical future patient population with 80% power to detect the differences in mean absolute error in Groups A and B, it is necessary to assume equal sample sizes in the two groups, since the ratio of their procedures A and B is not known. Based on this assumption, at least 86 procedures must be performed in each group to be able to detect a significant difference between the mean absolute error for the two methods A and B. Further statistical analysis revealed that, after reviewing the 49 recalibrated procedures, a 1° error correction was able to yield a significant improvement. The mean absolute error from 2.06 to 1.58, resulted in an improvement of accuracy from 91% to 98% using an accuracy window of ±3° with a power of at least 80%. If a different accuracy were to be desired, the number of cases would vary depending on the level of improvement sought (Table I).
While performing the procedures in Group A, the surgeon aimed for 0°, or neutral, mechanical alignment; however, the series of patients was found to exhibit an error of 1° excessive valgus. To re-aim or calibrate the surgeon/CAS team error, the subsequent group (Group B), was intentionally overcorrected manually in the alpha angle by 1° during femur resection. After 49 cases had been performed using this approach, the postoperative X-rays were analyzed for mechanical alignment to determine if this varus shift or recalibration of the target point, produced more accurate results. Both groups were treated identically in terms of exposure, surgical technique, clinical pathways, pain management, and follow-up routine.
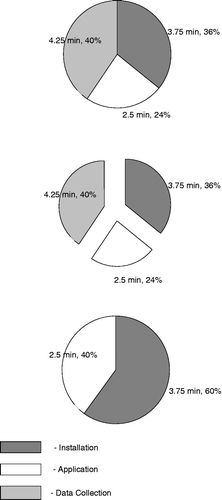
In addition to the accuracy assessment, intraoperative time segments considered attributable to the use of computer-assisted navigation were recorded to determine if the mental demands on the surgeon associated with re-targeting of the femur resection would result in extended procedure time. To investigate this in further detail, the CAS procedure was divided into three segments: CAS component installation, computation, and data collection. The installation segment included the installation and removal of trackers and waypoint acquisitions. The computation segment comprised the time devoted to verifying, analyzing and interpreting the data collected by the navigation system. The timing of this segment began with the screen view and ended with the start of the corrective measures by the surgeon; it did not include the actual manual correction time. For example, once a corrective osteotomy had begun, the time necessary to complete the new cut was not included in this segment. Finally, the data collection segment was defined as the time taken to collect measurements of ligament balance and knee kinematics for research and/or testing purposes.
Statistical analysis for continuous variables included the mean, standard deviation, and standard error of the mean. Confidence intervals for mean parameters were based on standard normal theory, and group comparisons are summarized by p-values corresponding to standard two-sample t-tests.
Table I. Estimated power for comparing Groups A and B based on the proportion of absolute errors of the AP mechanical axis (in degrees) greater than (or less than or equal to) the following cut-off values for accuracy level. This table relates the number of cases necessary to achieve a desired level of accuracy after recalibration has been completed. While the cases in Group B were based on the standard of ±3°, greater or lesser accuracy is possible.
Table II. Total mechanical alignment.
Table III. Absolute error of the AP mechanical axis for Groups A and B.
Results
Measurements on 36-inch X-rays showed that the mechanical alignment was correct within ±3° in 91% of Group A (Table I). The mean alignment was 1.21° valgus (95% Cl: 0.98, 1.44). Summary statistics for total mechanical alignment retrieved from both Group A and Group B are shown in Table II. The mean AP femoral angle (alpha) component alignment for Group A was −0.014° (95% Cl: −0.13, 0.11). The mean AP tibial angle (beta) alignment was 1.21° valgus (95% Cl: 1.01, 1.41). The mean sagittal tibial angle (gamma) alignment was 2.74° (95% Cl: −1.74, 0.60). All but the alpha angle represented satisfactory positioning for the surgical goal. With the persistence of the excessive valgus, the new recalibrated neutral alignment was re-aimed from the 0° of Group A to the new CAS angle alpha of the 1° varus in Group B. The data collected from Group A provides evidence that a level of accuracy greater than the desired ±3° is achievable. Significant improvement in accuracy was achieved in Group B by adjusting the femur resection (alpha angle) by 1° varus, thus affirming the hypothesis that the recalibration would yield a higher level of accuracy. Overall, alignment in Group B from summation of alpha and beta angles was correct to within ±3° in 98% of patients, with a mean alignment of 0.03° (95% Cl: −0.48, 0.54). The mean AP femoral angle (alpha) component alignment for Group B was −0.84° (95% Cl: −1.22, −0.46). The Group B mean AP tibial angle (beta) alignment was 0.86° valgus (95% Cl: 0.39, 1.33).
Table III gives the basic statistics for absolute error of the AP mechanical axis for both groups. The p-value from a two-sample t-test is 0.0016, which means the accuracy of Group B is significantly different from that of Group A with power of 0.896 (90%). This serves as an adequate sampling of how accuracy can be improved. There was no statistical difference in individual angles measured between groups if one eliminates the intentional varus target shift of the alpha angle. After changing the target values of the femur (new target = 1° varus), AP total alignment showed values closer to 0 in Group B than in the non-calibrated Group A.
Operative time from the incision to final wound closure ranged from 46.0 to 100.0 min, with a mean of 63.0 min and a standard deviation of 10.0 min, being similar in both groups. Procedure time was stabilized to an average of 58.0 min after performing the first 20 total knee replacements. In the time segment divisions there was no additional time burden noted for the mental computation in Group B. shows the mean time in minutes required to complete each established segment. For both groups, the installation took an average of 3.75 min (35% of the total computer navigated component of the procedure), computation an average of 2.5 min (25%), and data collection 4.25 min (40%).
Figure 1. The average duration of the computer navigated portion of the TKR procedures in this series. The data collection segment would not be needed in a non-research setting.
The average length of stay was 2.4 days, with a standard deviation of 0.66 days (range: 1.75–5.13 days). Patient complications included 4 manipulations, 4 infections, and 5 revisions, of which latter 4 were due to fractures and 1 to an over-sizing of the implant. All 4 fractures were in the same area of the medial plateau. There were 5 deep vein thrombosis, 2 pulmonary emboli, and 1 unrelated death due to a myocardial infarction.
Discussion
The purpose of this study was to evaluate the accuracy of the mechanical alignment in a series of total knee replacements and investigate how this can be improved in comparison to the literature by using the enhanced precision of CAS and recalibrating the resection angles (in this case those of the femur) to correct inaccuracy. Numerous articles have been published on the merit and value of CAS in achieving higher accuracy in TKA, as compared to a non-computer-assisted approach Citation[4], Citation[5], Citation[8–10], Citation[14–17]. While these studies appear to show conclusively that CAS improves accuracy of alignment, there is also an added time burden; not only because of the learning curve, but because in no study does the time to perform the procedure under CAS ever revert to neutral, or no additional time required.
There are studies showing significant advantages of MIS over traditional TKA with regard to postoperative total range of motion and total function scores Citation[18–20]. Conversely, others argue that MIS may be a causal factor for early failure in TKR, resulting from either reduced visualization or poor technique Citation[4], Citation[19]. While TKR failure may be multifaceted, it is generally agreed that a significant portion of failures evolve from malalignment, and that any improvement in this regard may have a beneficial effect on long-term survival Citation[20]. In no study, however, has CAS been shown to lead to worse results.
CAS is one means of achieving greater accuracy. Surprisingly, while manufacturers have mechanisms to calibrate many CAS systems before their release for use, there is no recognized method in which individual surgeons can calibrate or tune these systems. To date, no data is available regarding any attempt at making CAS more accurate than it already is. The present study was undertaken with the goal of determining if performing recalibration was logical and, if so, what the required time expense to the surgeons would be for this process.
Our initial 331 patients in Group A had reasonable accuracy, with 91% of the cases achieving a mechanical alignment within ±3° of a 0° target, compared to the average of four different series in the literature Citation[5], Citation[6], Citation[8], Citation[9] where an average of 93% (range 90–95%) of patients achieved this level of accuracy. A power analysis was performed to calculate the minimum number of cases needed to accept the outcome of improvement in accuracy in order to justify recalibration of the operative technique and this provided a starting point for a minimum acceptable accuracy. While the power analysis shows that the number of cases in Group A could be far smaller for a ±3° level of accuracy if there was a desire for a higher level of accuracy, this can only be accomplished by reviewing more cases. The overage of cases performed enabled construction of the accuracy-to-case ratio algorithm (Table I).
Additionally, it was intended to track this series of surgeries to monitor both the precision of both CAS and the surgeon so as not to be criticized for premature proclamations. Having observed no such drift, we feel confident in recommending that the surgeon assess their first 86 patients, which in this study resulted in a precision of ±3° in 91% of cases. If this number is met, a recalibration of the target point seems feasible. It should be noted, however, that sample size calculation is also dependent upon the precision standard deviations achieved by each individual physician.
Once the recalibration was performed, Group B was accurate in 98% of the cases, which was far better than anticipated by the original power study performed with Group A. While this can potentially be used in future non-research settings to assess whether or not a CAS system needs to be recalibrated, the results showed the test of the hypothesis to be a valid method for improving accuracy to levels not previously thought possible.
Time expenditures were evaluated as three defined segments attributed to adding CAS to a surgery: installation, computation, and data collection. All three segments showed no significant difference between the two groups (). Computation, which represents the bulk of the CAS procedures, had a mean duration of 4.25 min and is also the most important variable for several reasons. While the segments for installation and data collection become routine with little instance of difficulty or change, the computation can vary greatly from case to case, despite showing no statistical difference between the two groups. Some cases go smoothly, with accurate cuts being made the first time, while others prove more difficult. The addition of a new target did not cost or require more time; it merely became a single new target.
Several limitations were detected during the scope of the study. Clearly, the midterm assessment could be done with fewer patients than in our study. However, with our initial data, we did not have the ability to predict the higher level of precise accuracy that we may actually be able to achieve without having more cases to assess. Additionally, to ensure the series was stable, with no tendency for the CAS system to drift, the case series validated the dependability of the algorithm created. It was not imagined at the beginning of the study that the valgus shift would be so great. Sample size calculation is also dependent on the standard deviations achieved by each physician.
This study also had a secondary aim, which was to track time usage; however, the timed segments are somewhat subjective. While installation and data collection are routine and reproducibly timed, the computation can be extremely variable. The researchers relied solely on the surgeon verbally indicating when to start timing as a screen appeared on the CAS system, and timing stopped when the surgeon began a corrective surgical maneuver or the next screen was selected. The end time was then verbally agreed upon by the timer and surgeon. While this interpretation of data involves cognitive intellectual calculation of time along with motor reaction time, it is nevertheless an attempt to address the important issue of whether the recalibration of Group B cost additional time, which it did not. This study demonstrates the value of looking at CAS as an instrument that may enhance accuracy further than previously assumed. This may be achieved at an earlier timeframe, and should not result in any significant time increase for the procedure.
In summary, this study shows that accuracy derived from the use of CAS in TKR can be improved by using the precision gained by its consistency and applying it to a new recalibrated value. While our study demonstrates only mechanical axis improvement, there is no reason why this could not be applied to the entire field of angles in the continued effort to improve outcomes of TKR. This new target, or tuned CAS value, can be achieved at no additional time factor if the value is integrated into the surgeon's specific desired CAS value. While we did not change the software to a target value, it would be incumbent on the industry to provide such a customization mechanism specific to the CAS system and/or the surgeon.
Declaration of interest: The authors report no declarations of interest.
References
- Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop Relat Res 2005; 433: 152–159
- Tria AJ, Jr, Coon TM. Minimal incision total knee arthoplasty: Early experience. Clin Orthop Relat Res 2003; 416: 185–190
- Wülker N, Lambermont JP, Sacchetti L, Lazaró JG, Nardi J. A prospective randomized study of minimally invasive total knee arthroplasty compared with conventional surgery. J Bone Joint Surg Am 2010; 92: 1584–1590
- Barrack RL, Barnes CL, Burnett RS, Miller D, Clohisy JC, Maloney WJ. Minimal incision surgery as a risk factor for early failure of total knee arthroplasty. J Arthroplasty 2009; 24: 489–498
- Sparrmann M, Wolke B, Czupalla H, Banzer D, Zink A. Positioning of total knee arthroplasty with and without navigation support. A prospective, randomized study. J Bone Joint Surg Am 2003; 85: 830–835
- Victor J, Hoste D. Image-based computer assisted total knee arthroplasty leads to lower variability in coronal alignment. Clin Orthop Relat Res 2004; 428: 131–139
- Kanekasu K, Kondo M, Kadoya Y. Axial radiography of the distal femur to assess rotational alignment in total knee arthroplasty. Clin Orthop Relat Res 2005; 434: 193–197
- Kim SJ, MacDonald M, Hernandez J, Wixson RL. Computer assisted navigation in total knee arthroplasty: Improved coronal alignment. J Arthroplasty 2005; 20(7 Suppl 3)123–131
- Skowroński J, Bielecki M, Hermanowicz K, Skowroński R. The radiological outcomes of total knee arthroplasty using computer assisted navigation ORTHOPILOT. Chir Narzadow Ruchu Ortop Pol 2005; 70: 5–8
- Stulberg SD. How accurate is current TKR instrumentation?. Clin Orthop Relat Res 2003; 416: 177–184
- Bäthis H, Perlick L, Tingart M, Perlick C, Lüring C, Grifka J. Intraoperative cutting errors in total knee arthroplasty. Arch Orthop Trauma Surg 2005; 125: 16–20
- Tew M, Waugh W. Tibiofemoral alignment and the results of knee replacement. J Bone Joint Surg Am 1985; 67: 551–556
- Berry DJ. Computer-assisted knee arthroplasty is better than conventional jig based technique in terms of component alignment. J Bone Joint Surg Am 2004; 86(A11)2573
- Lionberger DR. The attraction of electromagnetic computer-assisted navigation in orthopedic surgery. Navigation and MIS in orthopedic surgery, J Stiehl, R Konermann, R Haaker, A DiGioia. Springer, Heidelberg 2007; 44
- Stulberg SD, Loan P, Sarin V. Computer-assisted navigation in total knee replacement: Results of an initial experience in thirty-five patients. J Bone Joint Surg Am 2002; 84-A(Suppl 2)90–98
- Matziolis G, Krocker D, Weiss U, Tohtz S, Perka C. A prospective, randomized study of computer-assisted and conventional total knee arthroplasty. Three-dimensional evaluation of implant alignment and rotation. J Bone Joint Surg Am 2007; 89: 236–243
- Lionberger DR, Weise J, Ho DM, Haddad JL. How does electromagnetic navigation stack up against infrared navigation in minimally invasive total knee arthroplasties?. J Arthroplasty 2008; 23: 573–580
- Kim YH, Sohn KS, Kim JS. Short-term results of primary total knee arthroplasties performed with a mini-incision or a standard incision. J Arthroplasty 2006; 21(5)712–718
- Tria AJ, Jr. Advancements in minimally invasive total knee arthroplasty. Orthopedics 2003; 26(8 Supp)s859–s863
- Tria AJ, Jr. Minimally invasive total knee arthroplasty: The importance of instrumentation. Ortho Clin North Am 2004; 35: 227–234