Abstract
Autologous fat grafting is an emerging and promising surgical technique in regenerative medicine, and its application is quickly spreading in plastic and reconstructive surgery of the breast. However, despite the advantages of the technique, surgical complications may occur, such as implanted tissue necrosis and resorption and onset of microcalcifications. In view of the hypothesis that the uniformity of the lipoaspirate transplantation is related to graft survival and a lower probability of complications, we developed an interactive lipomodeling planning software application based on a genetic algorithm that allows automatic optimization of the uniformity of fat tissue distribution. The input dataset consists of a 3D model of the patient's thorax, created from MRI scans, on which relevant structures are segmented. The developed software was tested starting from either an automatically generated plan or an initial guess of the optimal surgical plan, and in both cases the application yielded a consistent improvement in the planned fat tissue distribution by optimizing the position of the insertion points and the direction of the insertion pathways. On the basis of the simulations performed, the use of genetic algorithms for optimization of the geometry of autologous fat transfer in the breast proved to be effective. These results will foster further activities focused on the comparison of predicted optimized geometries and those obtained in real surgical cases as a means of obtaining a deeper knowledge of the potential influence of a uniform fat tissue distribution on the quality of the surgical outcome. The presented application is also put forward as representing a noteworthy step towards the clinical application of computer assisted planning tools in breast surgery.
Introduction
The grafting of autologous adipose tissue to restore acquired or congenital morphological defects is emerging as a promising surgical technique in regenerative medicine, and has found extensive application in plastic and reconstructive surgery of the breast Citation[1-7]. Use of the technique has also been described in a wide range of surgical applications, such as maxillo-facial surgery Citation[8-13], correction of Poland's syndrome Citation[14], Citation[15], correction of deformities in the gluteal-trochanteric region Citation[16], and healing of post-radiation tissue damage Citation[1].
The adipose tissue grafting procedure comprises three main steps: fat harvesting, fat preparation, and fat transplantation Citation[17]. Fat harvesting consists of liposuction of adipose tissue from the donor site (abdomen, flank, thigh or knee) by applying a vacuum to a syringe inserted in a small incision. The lipoaspirate is then prepared for transplantation through centrifugation to separate the adipocytes from blood, other cell types and cellular debris, and to produce a high concentration of adipose stem cells in the tissue suitable for transplantation, since these cells are ultimately considered responsible for the consistently observed regenerative processes Citation[18]. During the actual tissue transplantation in the breast, the purified lipoaspirate is grafted with a syringe into the subcutaneous and subglandular layers and into the pectoralis muscle along linear paths generated by the insertion of the syringe. The tissue is deposited as the syringe is withdrawn from the receiving sites, thereby generating a “net” of deposited lipoaspirate pathways from which the fatty tissue cannot diffuse due to breast tissue density.
Of the few side effects of the surgical procedure, tissue resorption and necrosis are the most frequently encountered Citation[7], Citation[19]. Tissue necrosis has been linked to the deposition of excessive quantities of lipoaspirate, concentrated in small volumes Citation[20]. This might arise from poor uniformity during tissue injection along each insertion pathway, as well as from overlaps and repeated crossing of the insertion pathways. The consequent reduced neo-vascularization in correspondence with grafted tissue accumulation has been considered responsible for the increased risk of tissue necrosis Citation[21], Citation[22], as well as for the formation of cysts and suspicious masses as revealed by post-operative control imaging Citation[23], Citation[24]. Insufficient vascularization in grafted tissue due to poor uniformity in deposition has also been indicated as the cause of tissue resorption, which can reach rates of up to 60% Citation[7], Citation[19], Citation[20], Citation[25].
In light of these observations, we proposed the hypothesis that technologies and methods of computer assisted surgical planning and intra-operative navigation Citation[26], Citation[27], designed to increase uniformity in lipoaspirate deposition, might represent valuable tools for enhancing the quality of surgical outcomes and reducing the risk of known side effects. For an application in breast autologous fat grafting, the basic requirement for pre-operative planning is the availability of a patient-specific volumetric model, derived from pre-operative imaging, on which a quantitative index describing the level of uniformity of the grafted tissue distribution can be optimized. From this perspective, the problem of uniformity is essentially one of geometrical optimization: the search for the best spatial configuration of the tissue deposition insertion and direction pathways on the patient-specific volumetric model of the receiving site. The output would be the set of spatial orientations and positions of the insertion pathways that maximizes the grafting geometrical uniformity by minimizing the volume of the region of interest untouched by the grafted tissue.
Such a problem is generally under-defined and ill-posed, with the number of solutions generally being very high (hypothetically infinite), even in presence of a set of geometrical and operative constraints. Among the several computational strategies that can be considered (least squares minimization, simulated annealing, etc.), we investigated the use of a large-scale optimization method based on a genetic algorithm Citation[28].
The algorithm was embedded in a clinically usable computer assisted planning application and a set of simulations was performed in order to investigate the contribution of the automatic quantitative optimization to the improvement in uniformity of fat deposition, within the framework of clinically feasible geometries. The results confirm the role that this tool could play in the surgical practice of autologous fat grafting in the breast, and highlight its current limitations as well as directions for future improvement.
Materials and methods
Dataset for surgical planning
The prerequisite for the planning of fat grafting in the breast is the availability of a volumetric dataset describing the patient's breast morphology to enable selection of the receiving site for the transplanted tissue. As a test dataset, we used T1-weighted magnetic resonance (MR) scans of the thoracic region of a patient, obtained using a 1.5-T Siemens Avanto scanner with a FLASH (Fast Low Angle SHot) MR sequence. The patient was scanned while lying prone, as the scanning was performed prior to a breast biopsy intervention. The thorax was scanned from the jugulum to the xiphoid process in the cranio-caudal (CC) direction, encompassing the whole body structure in the antero-posterior (AP) and latero-lateral (LL) directions. The voxel size was 0.8 × 0.8 × 1.8 mm for LL, AP and CC, respectively. The MR images acquired were then segmented semi-automatically using Amira© software (Visage Imaging GmbH, Berlin, Germany) to identify the anatomical structures that are relevant to quantitative surgical planning: the external surface, the fatty tissue, the mammary gland and the chest wall.
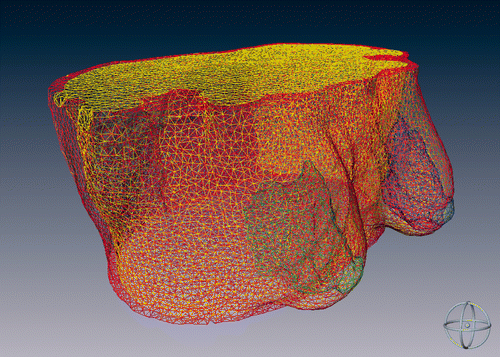
After the segmentation process, the created surfaces where triangulated and regularized, increasing the triangle dimensions, in order to achieve an appropriate trade-off between good anatomical representation and reduced computational cost for the planning algorithm (see ). A tetrahedral mesh was generated by means of a specific algorithm Citation[29] in order to create a volumetric representation of the anatomical structures and obtain a realistic 3D model of the patient's thoracic region. The mesh generation was tuned so that the tetrahedra volumes were consistently less than 40 mm3, and the ratio between the tetrahedron edge length and the tetrahedron circumsphere radius was less than 2. These constraints were applied to generate as regular a 3D mesh as possible.
Genetic algorithm
As mentioned above, the developed tool is based on a genetic algorithm, a well-known computational tool for the solution of large-scale optimization problems. The way in which a genetic algorithm works may be summarized as follows: Given a minimization problem and a population of similar (but not equal) individuals, namely the set of variables that represent candidate solutions to the problem, the optimal solution to the minimization problem is reached by the crossover of the individuals through succeeding generations and, eventually, by selecting “elite” individuals, i.e., individuals that are repeated identically in the consecutive generations. As the individuals that adapt better to the environment (i.e., solve the minimization problem) have more chances to reproduce themselves, after a limited number of generations the “best” individual set is found Citation[28],Citation[30].
In our implementation, the parameters provided as input to the algorithm are the external surfaces of the segmented structures (fat, mammary gland, chest wall) and, optionally, an initial surgical plan geometry defined interactively by the surgeon. The plan consists of the coordinates of the syringe insertion points and the preferred grafting pathway directions for each insertion point. Although a high level of graphic interaction is granted to the operator in the initialization phase (see Software implementation sub-section below), this phase can be omitted, as the application was implemented to work completely automatically. The initial plan, generated manually or automatically, represents the initial individual, from which the first generation of possible solutions to the optimization problem is created by adding Gaussian noise to the initial data. In addition, a set of geometrical constraints has been defined in order to confine the optimized solution within clinically applicable conditions. In particular, the algorithm discards solutions that envisage any of the following:
directions that superimpose on each other;
directions intersecting the mammary gland; and
directions outside the volume of interest.
As the “fitness” function, i.e., the merit index for ranking each individual belonging to each specific generation, the volume of untreated breast, namely the sum of tetrahedra of the volumetric patient-specific model untouched by the lipoaspirate deposition, was selected. This ensured maximization of the uniformity of the transplanted tissue distribution. To identify the voxels included in each insertion direction, each pathway was considered as a cylinder of the same size as the cannula used for insertion, surrounded by a cylindrical volume with the radius tunable by the operator. In this way, at each generation of solutions to the optimization problem, the amount of breast volume in contact with the grafted fat tissue can be calculated and the fitness function featured by each solution can be quantified.
In accordance with the strategy of genetic algorithms, each generation was treated by “natural selection” to select the best individuals. The sorting of the individuals on the basis of their fitness was followed by a ranking in order to give the best individuals a higher chance of being selected as parents of the following generation of solutions. Genetic crossover was implemented according to two different approaches: a uniform probability selection and a “roulette” probability selection. For the sake of clarity, this latter method assumes that the population is like a roulette wheel, in which each section width is proportional to the individual ranking, thus giving to “fit” individuals a higher chance of being selected. Random noise mimicking a “genetic mutation” to increase population variability was superimposed on a subset of individuals at each generation.
Software implementation
The developed software application was implemented in C++ using Qt (Nokia Norge AS, Oslo, Norway), VTK (Kitware, Inc., Clifton Park, NY) and Eigen as third-party libraries, in order to create a completely open-source software platform. Even though many open-source libraries are available for genetic algorithm implementation, an ad hoc library was written from scratch in order to optimize and speed up the surgical plan optimization. The application allows automatic calculation of the surgical plan after selection of the region of interest (ROI) on the volumetric patient-specific dataset and definition (manual or automatic) of the initial surgical plan geometry ().
Figure 2. Workflow of the lipomodeling planning software. (A) Contouring of the breast. (B) Region selection (blue mesh) and syringe entry points selection (cyan points). (C) Planned fatty tissue deposition direction on the ROI mesh (visualized as a wireframe).
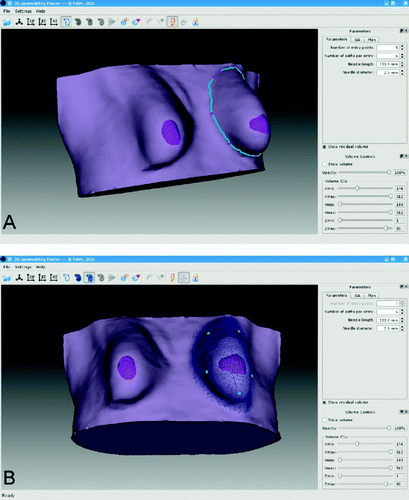
The ROI identification (A) is performed by manually tracing the ROI contour on the external surface to identify the surface mesh vertices delimiting the area of interest. The selected points are connected through geodesic functions that find the shortest path on the external surface running on mesh edges Citation[31]. Once the external contour has been created, the underlying model voxels are selected and a closed tetrahedralized volume is identified, within which the surgical plan is defined and the fitness function calculated.
In cases where manual surgical plan initialization is selected, the user is prompted to select the initial configuration of insertion points along the ROI contour (B), as well as the initial insertion pathways from each insertion point (C).
After initialization and before launching of the genetic algorithm, the user has the ability to define and tune the following parameters:
cannula length and diameter;
cannula minimum insertion depth (i.e., the minimum acceptable direction length);
number of individuals per generation;
maximum number of generations;
percentage of individuals of the succeeding generation created through crossover and simple genetic mutation; and
the algorithm used to define the crossover probability for each individual.
Surgical plan examples
In the following, examples of different options offered by the application for surgical planning are described.
Automatic surgical plan calculation
Under the fully automatic plan calculation option, an initial random configuration of insertion point positions and pathway directions is defined as the initial population. The insertion points are placed on the external surface of the contoured ROI, avoiding the nipple area and the underlying mammary gland. Initial directions are defined to fulfill the aforementioned constraints, thus never intersecting the mammary gland.
An output example of the automatic plan definition is shown in . The initial parameters were set as follows:
number of insertion points: 5
number of fat deposition directions: 5
cannula length and diameter: 130.0 × 2.0 mm
The genetic algorithm parameters were as follows:
maximum number of generations: 150
number of individuals per generation: 20
number of elite individuals: 2
crossover individuals: 80%
initial population variability: 3%
genetic mutation: 3%
genetic mutation after crossover: 1%
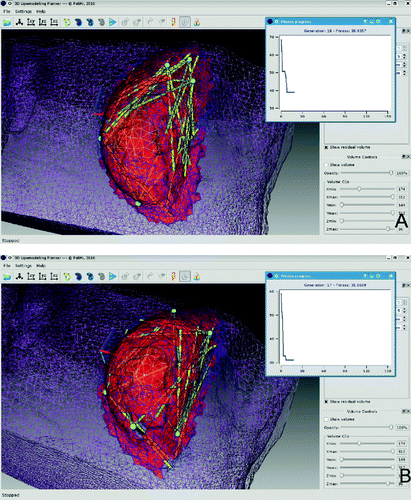
As shown in , the algorithm reaches a plateau in only 18 generations with a fitness value of 38.9 cm3, represented in the figure by the red volume. This final fitness value represents the overall volume of the voxels which are left untouched by the planned grafting geometry. As this represents only 28.9% of the total volume of the selected ROI, which was 134.4 cm3 (not including the mammary gland), this result is put forward as representative of a good performance of the optimization process. However, the good final fitness value is in contrast to the quality of the plan geometry, as three of the five insertion points are located in the upper breast quadrants, leaving the most non-treated volume in the lower medial quadrant. This condition requires a post-process correction of the surgical plan by the surgeon, who is able to move the insertion points and/or fat deposition directions to an under-treated region and re-run the genetic algorithm.
From this perspective, the automatic plan option of the application may be used as an initial step for the definition of an optimal plan that is reached after manual tuning of the initial automatic guess.
Custom surgical plan definition
The custom surgical planning option is provided to allow the user to define the initial configuration of the surgical plan geometry in terms of the position of the insertion points and the direction of the insertion pathways.
B shows a custom plan calculation output. The parameters used are the same of those described in the previous sub-section, the only difference being the initial position of the insertion points: here, three were defined in the lower quadrants and two in the upper ones. The final fitness value of 31.1 cm3 (23.1% of the total ROI volume) is lower than in the previous example and is reached in 17 generations.
As shown in B, the better distributed initial points and pathway directions resulted in the untreated volume being concentrated in a superficial tissue layer, a much more uniform distribution of fat deposition directions, and, consequently, a quicker and better algorithm convergence. Also, in this case the application allows the user to modify the calculated plan in order to improve the resulting geometry within the framework of a multi-stage optimization process.
Discussion
In recent years, autologous fat grafting has become a valid alternative in plastic and reconstructive surgery of the breast. The geometry of fat tissue deposition within the receiving area is believed to represent a crucial element in maximizing the surgical outcome and minimizing complications, such as cysts, fat necrosis, and tissue resorption. Within this framework, this paper has presented a simple and user-friendly computer assisted surgical planning tool capable of quantitatively and automatically optimizing the grafting geometry by means of a genetic algorithm large-scale optimization procedure working on a 3D model of the patient. The implemented application offers automatic as well as manual initialization options, and allows the user to refine the resulting surgical plan geometry to correct eventually under-treated sub-volumes of the selected region of interest, exploiting the high level of human-computer interaction provided by the application. Clinical applicability is ensured by the high degree of flexibility in defining geometrical constraints such as off-limits sub-volumes and limits on insertion point position and pathway direction.
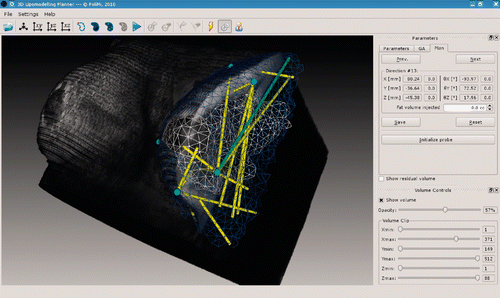
Tuning of the genetic algorithm parameters was made possible to enable the user to control the way in which the optimization procedure explores the space of the possible solutions. This option lends itself as an opportunity to trade off the speed of algorithm convergence against the width of the solution hyperspace being explored by the optimization process. The superimposition of the volumetric imaging dataset on the segmented structures allows the user to benefit from a composite representation, facilitating a more clinical interpretation and evaluation of the quality of outcome of the optimization ().
We are aware that the reported results are severely limited by the use of only one volumetric dataset acquired from a prone patient for plan optimization. For this reason, the study is put forward as a “proof of principle” of the use of large-scale optimization algorithms for surgical plan optimization in breast autologous fat grafting. There is no question that further validation activities are required to assess the usability and functionality of the implemented computational planning tool. This evaluation can be performed by comparing non-optimized and optimized plans on a large volumetric dataset derived from several patients, for whom volumetric models are available from pre-operative imaging, in which the breast anatomy is representative of the intra-operative scenario. In breast autologous fat grafting, the volumetric dataset should be acquired with the patient in a position similar to that assumed during surgery (typically a semi-sitting position). This requirement not only applies to optimization of the surgical geometry on a realistic patient volumetric model, but is also mandatory from the perspective that computer assisted surgical planning must be complementary to systems for intra-operative navigation that are capable of providing real-time indications for surgical guidance. The accuracy with which the indications of the treatment plan can be transferred to the reality of the surgical intervention depends strictly upon the quality of the registration procedure, which takes into account the reliability of the patient anatomical representation in the plan and in the surgical room as a crucial prerequisite. Unconventional patient positioning in the MR scanner, as well as the use of an MR atlas coupled with intra-operative optical surface scanning Citation[32], Citation[33] or ultrasound imaging and deformable modeling morphing Citation[34], Citation[35], Citation[36], are possible approaches to addressing this question and merit specific investigations for clinical feasibility and validation.
Despite these still-pending issues, we feel confident that the described method and related software application might already represent a useful tool for providing surgeons with guidelines for optimizing the geometrical design of the surgical procedure on representative volumetric models of the patient, as well as for their training in the emerging technique of autologous fat transfer in the breast. Ultimately, we believe that the application of computer assisted planning and intra-operative navigation is the only way to obtain evidence of the effect of geometrical uniformity in fat tissue deposition on the quality of surgical outcome and the rate of complications, and will thus contribute to an increase in the therapeutic efficacy of the technique.
Declaration of interest: The authors have no real or perceived financial or personal conflicts of interest relating to the presented work.
References
- Rigotti G, Marchi A, Galiè M, Baroni G, Benati D, Krampera M, Pasini A, Sbarbati A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: A healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg 2007; 119: 1409–1422
- Rigotti G, Marchi A, Sbarbati A. Adipose-derived mesenchymal stem cells: past, present, and future. Aesthetic Plast Surg 2009; 33: 271–273
- Auclair E. Benefit of complementary lipofilling in aesthetic breast augmentation with implant. Ann Chir Plast Esthet 2009; 54: 491–495
- Fitoussi A, Pollet A, Couturaud B, Salmon RJ. Secondary breast reconstruction using exclusive lipofilling. Ann Chir Plast Esthet 2009; 54: 374–378
- Zocchi ML, Zuliani F. Bicompartmental breast lipostructuring. Aesthetic Plast Surg 2008; 32: 313–328
- Rigotti G, Marchi A, Stringhini P, Baroni G, Galiè M, Molino AM, Mercanti A, Micciolo R, Sbarbati A. Determining the oncological risk of autologous lipoaspirate grafting for post-mastectomy breast reconstruction. Aesthetic Plast Surg 2010; 34: 475–480
- Delay E, Garson S, Tousson G, Sinna R. Fat injection to the breast: Technique, results, and indications based on 880 procedures over 10 years. Aesthet Surg J 2009; 29: 360–376
- Barret JP, Sarobe N, Grande N, Vila D, Palacin JM. Maximizing results for lipofilling in facial reconstruction. Clin Plast Surg 2009; 36: 487–492
- Schmitz S, Weis C, Morley S, Demey A, Dabernig J. Treatment of facial lipodystrophy syndromes. lipofilling versus free flap surgery. Eur J Plast Surg 2008; 31: 305–310
- Orlando G, Guaraldi G, De Fazio D, Rottino A, Grisotti A, Blini M, De Santis G, Pedone A, Spaggiari A, Baccarani A, et al. Long-term psychometric outcomes of facial lipoatrophy therapy: Forty-eight-week observational, nonrandomized study. AIDS Patient Care STDS 2007; 21: 833–842
- Hoehnke C, Eder M, Papadopulos NA, Zimmermann A, Brockmann G, Biemer E, Kovacs L. Minimal invasive reconstruction of posttraumatic hemi facial atrophy by 3D computer-assisted lipofilling. J Plast Reconstr Aesthet Surg 2007; 60: 1138–1144
- Cortese A, Savastano G, Felicetta L. Free fat transplantation for facial tissue augmentation. J Oral Maxillofac Surg 2000; 58: 164–169
- Clauser LC, Tieghi R, Consorti G. Parry-Romberg syndrome: volumetric regeneration by structural fat grafting technique. J Craniomaxillofac Surg 2010; 38: 605–609
- Pinsolle V, Chichery A, Grolleau JL, Chavoin JP. Autologous fat injection in Poland's syndrome. J Plast Reconstr Aesthet Surg 2008; 61: 784–791
- Delay E, Sinna R, Chekaroua K, Delaporte T, Garson S, Toussoun G. Lipomodeling of Poland's syndrome: A new treatment of the thoracic deformity. Aesthetic Plast Surg 2010; 34: 218–225
- Valeriani M, Mezzana P, Terracina FSM. Liposculpture and lipofilling of the gluteal-trochanteric region: Anatomical analysis and technique. Acta Chir Plast 2001; 43: 95–98
- Rosing JH, Wong G, Wong MS, Sahar D, Stevenson TR, Pu LLQ. Autologous fat grafting for primary breast augmentation: A systematic review. Aesthetic Plast Surg 2011; 35: 882–890
- Philips BJ, Marra KG, Rubin JP. Adipose stem cell-based soft tissue regeneration. Expert Opin Biol Ther 2012; 12: 155–163
- Niechajev I, Sevcuk O. Long-term results of fat transplantation: clinical and histologic studies. Plast Reconstr Surg 1994; 94: 496–506
- Carpaneda CA, Ribeiro MT. Percentage of graft viability versus injected volume in adipose autotransplants. Aesthetic Plast Surg 1994; 18: 17–19
- von Heimburg D, Lemperle G, Dippe B, Krüger S. Free transplantation of fat autografts expanded by tissue expanders in rats. Brit J Plast Surg 1994; 47: 470–476
- Mandrekas AD, Assimakopoulos GI, Mastorakos DP, Pantzalis K. Fat necrosis following breast reduction. Br J Plast Surg 1994; 47: 560–562
- Pierrefeu-Lagrange AC, Delay E, Guerin N, Chekaroua K, Delaporte T. Radiological evaluation of breasts reconstructed with lipomodeling. Ann Chir Plast Esth 2006; 51: 18–28
- Chala LF, de Barros N, de Camargo Moraes P, Endo E, Kim SJ, Pincerato KM, Carvalho FM, Cerri GG. Fat necrosis of the breast: Mammographic, sonographic, computed tomography, and magnetic resonance imaging findings. Curr Probl Diagn Radiol 2004; 33: 106–126
- Zheng DN, Li QF, Lei H, Zheng SW, Xie YZ, Xu QH, Yun X, Pu LL. Autologous fat grafting to the breast for cosmetic enhancement: Experience in 66 patients with long-term follow up. J Plast Reconstr Aesthet Surg 2008; 61: 792–798
- Desai A, Dramis A, Kendoff D, Board TN. Critical review of the current practice for computer-assisted navigation in total knee replacement surgery: Cost-effectiveness and clinical outcome. Curr Rev Musculoskelet Med 2011; 4: 11–15
- Inamura K, Lemke HU. Technology and its clinical application in the field of computer-assisted radiology and surgery. Biomed Imaging Interv J 2007; 3(3)e41
- Goldberg DE. Genetic Algorithms in Search, Optimization and Machine Learning. Addison Wesley, Boston, MA 1989
- Si H. Adaptive tetrahedral mesh generation by constrained Delaunay refinement. Int J Numer Meth Eng 2008; 75: 856–880
- Riboldi M, Baroni B, Spadea MF, Tagaste B, Garibaldi C, Cambria R, Orecchia R, Pedotti A. Genetic evolutionary taboo search for optimal marker placement in infrared patient set-up. Phys Med Biol 2007; 52: 5815–5830
- Cormen TH, Leiserson CE, Rivest RL, Stein C. Introduction to Algorithms. 2nd edition. MIT Press and McGraw-Hill, Boston, MA 2001
- Patete P, Riboldi M, Spadea MF, Catanuto G, Spano A, Nava M, Baroni G. Motion compensation in hand-held laser scanning for surface modeling in plastic and reconstructive surgery. Ann Biomed Eng 2009; 37(9)1877–1885
- Patete P, Eder M, Raith S, Volf A, Kovacs L, Baroni G. Comparative Assessment of 3D Surface Scanning Systems in Breast Plastic and Reconstructive Surgery. Surg Innov 2012, In Press. DOI: 10.1177/1553350612463443.
- Azar FS, Metaxas DN, Schnall MD. Methods for modeling and predicting mechanical deformations of the breast under external perturbations. Med Image Anal 2002; 6: 1–27
- Chung JH, Rajagopal V, Nielsen PME, Nash MP. A biomechanical model of mammographic compressions. Biomech Model Mechanobiol 2008; 7: 43–52
- Patete P, Iacono MI, Spadea MF, Trecate G, Vergnaghi D, Mainardi LT, Baroni G. A multi-tissue mass-spring model for computer assisted breast surgery. Med Eng Phys 2012, In Press. DOI: 10.1016/j.medengphy.2012.03.008.