Abstract
Tumors in the pelvic region cause deformation and destruction of bony structures. Because the original pelvic anatomy cannot be adequately assessed at the tumor site, reconstruction with patient-specific implants is required. A widely used strategy for the reconstructive planning is mirroring of the contralateral side. We analyzed the statistical shape model (SSM)-based reconstruction method and compared it with the mirroring approach. Our approach used a gender-specific pelvic SSM (n = 50 for each gender) to generate implant geometries. The main objectives of this study were to analyze and evaluate the virtual anatomical reconstruction of eight tumor-damaged pelvic bones using the SSM approach. We achieved an overall mean deviation distance of 0.89 mm and 1.26 mm for the reconstruction of the equivalent defect in the healthy hemipelvis. Quantitative comparison with the mirroring method showed that the SSM-based reconstruction method reconstructs the defect with the same clinically acceptable accuracy as the mirroring method. The study demonstrates that the presented model can be a valuable tool for the planning of pelvic reconstructive surgery and implant design.
Introduction
Approximately 12–20% of all primary bone sarcomas are localized to the pelvis Citation[1]. The pelvis has a complex anatomy that includes different bone structures and a unique shape. Thus, preservation of the biomechanical characteristics of the pelvic ring after resection of large parts of the pelvis (particularly around the acetabulum) due to malignant tumors is very challenging Citation[2]. The current concepts used to attempt preservation include reconstruction with extracorporeal irradiated pelvic bone and re-implantation Citation[3], homologous massive bone grafts (allografts) Citation[4–6], pelvic megaprostheses Citation[7–9], and transposition of the hip to the sacrum Citation[10]. Long-term reliability and functionality after limb salvage surgery in pelvic sarcoma patients can best be achieved by near-anatomic reconstruction of the affected bone Citation[3]. However, long-term results are often less than ideal because the artificial and homologous transplants used to reconstruct the resected osseous part of the pelvis do not resemble the bone they are replacing sufficiently closely to achieve a near-anatomic reconstruction Citation[11], Citation[12].
The preoperative diagnostics of a pelvic tumor include MRI and CT scans. This imaging reveals the extent of the tumor and the damage to the pelvis. As the tumor usually causes deformation and destruction of bony structures, the original anatomy of the pelvis cannot be adequately assessed at the tumor site. A good strategy for planning the reconstruction of the resected part of the pelvis is mirroring of the contralateral side Citation[13–15]. This enables the fast generation of a model that is anatomically similar to the target region.
The goals of reconstruction with an implant are to bridge the destroyed bone, to enable the fast integration of the implant, and to stimulate the generation of autologous new bone in the resected area. These aspects are related to the shape of the implant, its material, and the mechanical response produced. Currently, this is probably the only option offering the potential for long-term full functionality of the involved extremity. All of these factors must be taken into consideration when designing an orthopedic implant as a replacement for a major part of the pelvis.
In this study, we analyzed a new planning method for the reconstruction of pelvic defects. Our approach uses pelvis statistical shape models (SSMs) Citation[16] to generate patient-specific implant geometries. The parametric pelvis model can be altered (via a unique set of transformation parameters) toward an optimal match with a patient's pelvic anatomy Citation[17].
Generation of SSMs for the pelvis has already been reported in a few papers from other groups, but they focused primarily on segmentation applications Citation[18–22]. To our knowledge, only the work of our group Citation[23–25] has been concerned with SSM-based pelvic reconstruction approaches (that is, the reconstruction of resected, non-existing parts of the bone). The main objectives of the present study were to present and evaluate the virtual anatomical reconstructions of eight tumor-damaged pelvic bones using gender-specific pelvis SSMs. An important aspect of this evaluation was the comparison with the traditional treatment method, i.e., mirroring of the contralateral side.
Material and methods
Patients and model dataset
Eight patients had pelvic tumors that required wide resection and consequent reconstruction. Patient data is summarized in . The acetabular region was involved in the resection in five cases (cases 2–5 and 7), while in the first case the tumor was restricted to the pubic bone and ischium. The tumors in the sixth and eighth cases were localized to the os ilium.
Table I. Patient data.
Our planning approach used the CT dataset for each patient and the gender-specific pelvic SSM for optimal reconstruction of the morphological region of interest. For the generation of the pelvic SSM, a CT data pool including over 150 pelvic CT datasets was collected (average voxel size: 0.7 × 0.7 × 1.25 mm). From the 152 processed pelvises 32 female and 20 male pelvises were excluded. The exclusion criteria were a history of hip dysplasia, fracture or prosthetic replacement, and datasets that did not include the entire pelvic anatomy in the scanner's field of view. The rest of the CT data underwent the bone segmentation process until we obtained segmented pelvic bone surfaces from 50 male and 50 female subjects. Thus, we were left with 100 datasets for generation of the pelvic SSM.
Pelvic SSM
The geometrical data of the pelvises (called the training set) were obtained via segmentation and surface processing methods Citation[26]. The different stages of the data processing pipeline used for the generation of the pelvic SSM are presented in . The CT dataset was subdivided into male (n = 50) and female (n = 50) pelvises, and from each training set a gender-specific SSM was generated.
Figure 1. Stages of the gender-specific pelvis SSM generation pipeline. The SSM generation procedure consists of the following consecutive steps: segmentation of pelvic bone structures from the collected CT datasets; generation of the male and female training sets; mesh alignment based on Procrustes analysis; and, finally, application of principal component analysis (PCA) to obtain the gender-specific SSM.
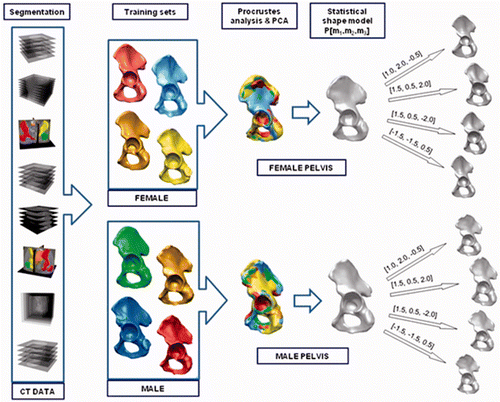
The SSM method had four main stages: segmentation, surface mesh processing, data alignment, and principal component analysis Citation[27], Citation[28]. To enable better understanding and analysis of the applied reconstruction method, the description from our previous papers Citation[23–25] is reiterated below, supplemented with more precise details of the implementation.
In our approach, the pelvic shapes were described by triangular mesh surfaces. The generation of the training set was accomplished via segmentation of all collected CT data. In our self-developed segmentation, visualization, and modeling software, SeVisMo Citation[23], various segmentation methods have been implemented to identify and separate the required osseous structures in the pelvic region.
After the segmentation stage, the triangular mesh surfaces underwent special processing and optimization. The surface mesh representation of the segmented bones was generated by the Marching Cubes algorithm Citation[29]. As CT datasets can contain imaging artifacts (e.g., ring artifacts, beam-hardening effects, motion artifacts or partial volume artifacts) or low-contrast images Citation[30], the following surface mesh processing methods were required to improve the surface mesh quality: cleaning, smoothing, remeshing, and decimating. At this stage in the processing, in addition to the data-processing software that we developed ourselves, the RapidForm commercial surface-processing and reverse-engineering software (INUS Technology, Inc., Seoul, Korea) was used Citation[31].
At the next stage of the data processing pipeline, a relationship between the different shapes of the pelvis had to be established for the statistical analysis. First, the number of nodes in all anatomically corresponding meshes of the training set had to be adjusted. We achieved this by using the thin-plate spline transformation Citation[32] as previously described Citation[23–25].
Once the relationship between the different shapes from the training set had been established, a Procrustes analysis and principal components analysis (PCA) was performed Citation[27], Citation[28], and a pelvic statistical shape model was obtained, along with the most dominant modes of variation (see ). The implementation of the described pipeline stage in SeVisMo was based in part on the VTK library Citation[33]. The mathematical definition of the pelvis SSM P is as follows:where P0 is the mean pelvic shape model representation and φk are the principal modes of variation (eigenvectors) Citation[34–36]. Changing the deformation parameters Dk (shape weights) allowed us to obtain a new model instance, which is a deformed version of the mean model P0. Different instances of both gender-specific pelvic SSMs for the first three modes (those responsible for the most significant pelvic geometry deformations) are presented in . The use of a robust non-deterministic optimization method, called evolutionary algorithms, allowed us to identify the mode vector that defined a model instance that optimally matched the pelvic surface to be reconstructed Citation[37].
The following objective function C is minimized in our optimization problem:where [xs(i), ys(i), zs(i)] denotes the i-th mesh point on the surface S to be reconstructed, [xp(i), yp(i), zp(i)] is the closest point on the surface P which is an instance of SSM, and n denotes the number of mesh points on the reconstructed surface S.
We built gender-specific statistical hemipelvis models from the training pelvis mesh surfaces of 50 females and 50 males. The generated pelvic SSM allowed estimation of the anatomical target geometry for the reconstruction of any region of interest.
Surgical planning based on this SSM was applied to find the anatomically correct bone shape for reconstruction of the pelvis after tumor resection in eight cases. Surgeon-guided virtual pelvic osteotomy was performed for each surface mesh by cutting away the mesh part corresponding to the pathological region. The reconstruction quality evaluation was based on the discrete probabilistic distribution of distances between surface mesh nodes, and was characterized in terms of means, standard deviations (SD), and minimum and maximum values. Statistical analysis was performed with SPSS software version 11.5 (SPSS Inc., Chicago, IL) and Excel (Microsoft, Redmond, WA).
Results
The volumetric characterization of all male and female pelvises used for the generation of the model is presented in .
Table II. Volumetric characterization of the hemipelvises in the gender-specific pelvic SSMs.
The overall mean volume of the male hemipelvis was 498 cm3 for the right side and 497 cm3 for the left, whereas the corresponding volumes for the female hemipelvis were 374 cm3 and 371 cm3, respectively. There were no significant volume differences between the right and left sides for either gender (p = 0.855). However, we found a significant difference between female and male pelvic volumes (p < 0.001).
An interesting question was how much differentiation occurs within the left and right hemipelvis of an individual, taking into account linear distances between important pelvis-specific landmarks. We calculated the distance between the anterior superior iliac spine (ASIS) and the center of a sphere inscribed in the acetabulum for all pairs of hemipelvises in both training sets. The results, presented in , show the existence of significant asymmetry, at least for the measured distance (the maximal difference in distance between the left and right side was 16.6 mm). In such cases, pelvic reconstruction by the mirroring method would be unsatisfactory from the medical point of view. The percentage of the hemipelvis surface to be reconstructed for all eight cases is presented in .
Table III. Statistical view of the distance between the anterior superior iliac spine (ASIS) and the center of the sphere inscribed in the acetabulum for all pairs of hemipelvises (male and female) in both training sets.
Table IV. Percentage of hemipelvis surface to be reconstructed.
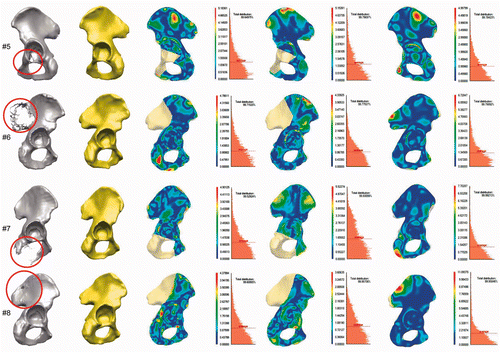
All of the clinical cases used in our evaluation of the model's reconstruction ability are presented in . The estimated mean distance and the standard deviation between the preoperative pelvic surface in the intact area and the reconstructed surface are illustrated for each case in . We achieved an overall mean deviation distance of 0.89 mm (min distance = 0.000024 mm; max distance = 6.99 mm) for the intact area and 1.26 mm for the reconstruction of the equivalent defect in the healthy hemipelvis (for the whole hemipelvis area) (see ).
Figure 2. Application and evaluation of the SSM-based pelvic reconstruction approach in clinical cases. First column: Location of the defects (tumors) in the segmented CT data, marked with red circles. Second column: Reconstructed hemipelvis surface (shown in yellow). Third column: Color-coded map of the distances between the original and the reconstructed pelvic surfaces. The uncolored portion is the reconstructed defect area (after virtual wide resection of the bone tumor). Fourth column: Distribution of the distances between the original and the reconstructed (SSM-based) pelvic surfaces in the intact areas, and the average distance (red dotted line). Fifth column: Map of distances between the original and the mirroring-based reconstructed pelvic surfaces (in intact areas). Sixth column: Distribution of the distances for the reconstruction method in the preceding column. Seventh column: Distance maps for the SSM-based reconstruction of the equivalent osteotomy performed in the contralateral healthy hemipelvis (for the whole hemipelvis). The contralateral healthy hemipelvis acts as the ground truth in this part of the evaluation. Eighth column: Distribution of the distances for the reconstruction method in the preceding column.
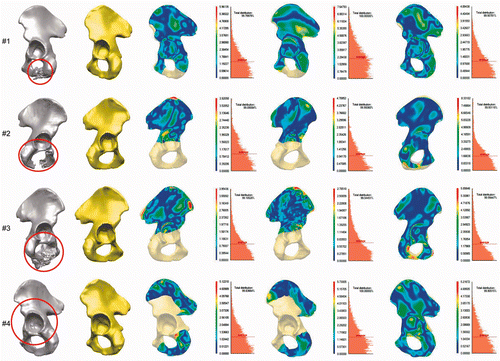
Table V. Mean distances from original pelvis to SSM-based reconstruction and mirroring method-based reconstruction for all evaluated cases (intact area only).
Table VI. Mean distance differences for SSM-based reconstruction and mirroring method-based reconstruction for each case and degree of improvement.
Table VII. Mean distances from contralateral healthy hemipelvis to SSM-based reconstruction of equivalent osteotomy performed in contralateral healthy hemipelvis (presented separately for intact area only, defect area only, and whole hemipelvis area).
At the optimization stage, the objective function C (Equation 2) was optimized and the mode vector giving the best reconstruction estimated. To preserve 98% of the whole pelvic shape information, we optimized the objective function in the n-dimensional feasible region (n = 37 for the male SSM and n = 38 for the female SSM). An evolutionary algorithm called evolution strategy (μ + λ) was chosen at this stage Citation[5], where μ denotes the size of the parent population and λ the size of the offspring population from which individuals are selected. Offspring consisted of 100 individuals with 15 elitists. The uncorrelated Gaussian mutation with n step sizes and mutation strength σ = 0.1 was selected Citation[38]. Recombination was provided by the discrete recombination for object variables and the global intermediate recombination for the step size.
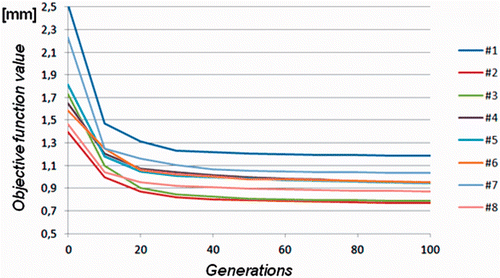
The algorithm's stop condition is fulfilled after 300 iterations or repetition of the same result in 5 consecutive offsprings. In most cases, satisfactory results were obtained after 80 generations. The convergence plots for all reconstruction cases (see ) reveal very good convergence behavior for the evolutionary algorithm.
Figure 3. Convergence behavior of the objective function during the evolution strategy-based optimization process for each reconstruction case. Equilibrium is achieved after 80 generations, on average.
For comparison purposes, we also reconstructed the pelvises using the mirroring method (i.e., a defect hemipelvis was reconstructed using the mirrored contralateral healthy hemipelvis and matched to the intact area). For the matching of the surface meshes we used two surface matching methods: the chamfer distance-based approach Citation[39] and the Iterative Closest Point (ICP) method Citation[40]. The transformation model was a rigid-body transformation. In all cases, both methods delivered almost the same matching transformation, giving for the optimal objective function value a difference at a level of 0.02 mm. For this reason, presents the estimated values for one matching method only, i.e., chamfer matching.
presents the mean distance differences for the SSM-based reconstruction and the mirroring method-based reconstruction for each of the eight clinical cases and the corresponding degree of improvement. Positive differences for the estimated mean distances indicate the superiority of the SSM-based method in those cases; negative values mean the mirroring method was better. Thus, for our eight selected clinical cases, the mirroring-based pelvis reconstruction method gave better results than the SSM-based reconstruction method in two cases, while the SSM-based reconstruction method was better in six cases.
In addition, we compared the SSM-based reconstruction of the equivalent osteotomy when performed in the contralateral healthy hemipelvis (for intact areas and defect areas separately, as well as for the whole hemipelvis). The contralateral healthy hemipelvis acted as a ground truth in this part of evaluation. The results of this evaluation are presented in .
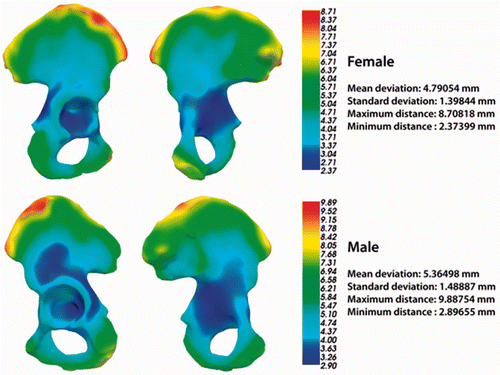
For a better understanding of different aspects of pelvic reconstruction quality, pelvic shape variability maps were calculated based on the pelvic surfaces belonging to both training sets (). They show, for the mean pelvis models (both male and female), the color-coded average deviation from each surface of the training set. The mean pelvis model is defined as the pelvis statistical shape model in which all mode parameters are equal to zero. From the variability maps it can be seen that certain regions deviated more than the rest of the pelvic surface. These regions were the iliac crest region, the sacroiliac region and the pubis region.
Figure 4. Pelvis shape variability maps based on the male and female pelvic surfaces from both training sets.
We have also prepared a short animation showing in three dimensions some aspects of the SSM-based reconstruction and matching process, as well as two short animations showing, again in three dimensions, the pelvis shape variability maps for the gender-specific training sets. These animations may be viewed at the following URLs:
Discussion and conclusions
The use of anatomical and statistical shape modeling is currently an area of intense investigation. Several groups have developed SSMs for various anatomical structures, including vertebrae Citation[41], the mandible Citation[42], the femur and pelvis Citation[43], the rib cage Citation[44] and the pelvis alone Citation[18], Citation[25]. For more examples of the use of SSMs in medical image processing, refer to the review by Heimann and Meinzer Citation[17]. In the present study, we investigated the strength and accuracy of a reconstruction method based on a pelvic SSM in eight realistic clinical cases. Calculating the mean distance between the original and reconstructed pelvic surfaces (in the non-defect area only, because of the lack of a ground truth) allowed us to estimate the overall alignment quality. In addition, the reconstruction of the defect was based not only on the geometrical alignment, but also on the statistical fit of the gender-specific normal anatomy-oriented model. We found that the reconstruction quality, measured as the surface deviation between the pelvic surfaces in the intact area, was of a very high level when this method was used, as we obtained a mean distance of only 0.89 mm in the eight cases. The reconstruction quality in the experiments with the ground truth (see ) achieved a mean distance of 1.26 mm for the whole hemipelvis reconstruction, while the average reconstruction quality for the defect area was only 1.72 mm. It can be observed that the sacroiliac region of the ilium (case 6) and the pubis bone (case 3) are the regions where the traditional mirroring approach is better than SSM-based reconstruction. The probable reason for this is the highly individual or highly variable shape of these areas, as can be seen in . The diverse anatomical variants of the sacroiliac joints were described by Prassopoulos et al. Citation[45]. The omission of some variants from the training set could negatively influence the method's reconstruction ability for this region.
One additional aspect of the reconstruction accuracy should be taken into account. This is the discrepancy between the average age of the pelvises in our training sets (the average male age was 62.7 years [SD = 13.6, min = 20, max = 83] and the average female age was 65 years [SD = 14.7, min = 26, max = 95]) and the age of the pelvises to be reconstructed (see ): five patients were under 43 years old and the other three were aged 56, 59 and 59 years. This could be an important factor which has a negative influence on the reconstruction accuracy for the SSM-based method.
To our knowledge, there has been no previous study of a pelvic defect reconstruction method based on gender-specific SSMs. Our results obtained using the SSM-based surgical planning method indicate that it can be used successfully to virtually reconstruct the resected pelvic bone in a manner that strongly correlates with the anatomical shape of the area of interest. Our previous reconstruction approaches began with the mirroring method. One of the disadvantages of the mirroring method is the natural asymmetry in human anatomy Citation[46–49]. An additional limitation is that the mirroring method can only be applied to unilateral defects. Consequently, multifocal defects remain a challenging problem in reconstructive pelvic surgery planning.
The performed evaluation gives us a quantitative comparison of the reconstruction quality by comparing the SSM-based and mirroring method-based approaches for the eight clinical cases. Our results show that a reconstruction using the gender-specific pelvis SSM was better than a reconstruction using the mirroring method in six cases, and worse in two cases.
In the context of intraoperative matching of pelvis surfaces, Barratt et al. 2008 Citation[43] used a pelvic SSM (based on CT data) for reconstruction of an anatomical pelvic surface acquired with only three-dimensional ultrasound imaging (3D USG). They estimated that the average root mean square distance between the ultrasound-derived surface and the reconstructed one was 3.5 mm over the whole bone and 3.7 mm in the region of surgical interest.
Seim et al. generated a pelvic SSM for CT segmentation purposes (using 50 CT datasets of mixed gender in the training set). Using a leave-one-out alignment experiment, they achieved an average surface distance of 1.2 mm. A better matching of the segmented surface to the reference surface (0.7 mm) can be achieved using an additional free-form deformation step Citation[22]. To the best of our knowledge, the approach of Seim et al. is the best pelvic segmentation method using the pelvic SSM.
The evaluation performed in the present study indicates that our SSM-based defect reconstruction method is comparable in terms of accuracy with the best SSM-based reconstruction method for segmentation purposes. However, it must be noted that the application areas and goals of the bone defect reconstruction method are different from those of the segmentation-oriented reconstruction methods, and more extensive evaluation of both approaches is needed.
Allografts are still used to reconstruct periacetabular defects, and the matching of massive allografts remains problematic Citation[4]. Even with a large bone bank, a perfect-fit allograft is difficult to find Citation[50], because only a discrete number of pelvic shapes are available. Paul et al. analyzed a 3D registration method for matching the anatomy of the allograft with that of the recipient Citation[15]. They assessed the superiority of the voxel intensity-based 3D alignment method over the 2D template method, and found that the selection of the best matching allograft was improved with the 3D method. A direct comparison of our approach with this method is not feasible because the latter's measurements of similarity are voxel-based (accounting for the intensity values of the CT data voxels), while our method is surface-based (accounting for the distance between bone surfaces).
In addition, the fitting accuracy of a preoperatively produced custom-made prosthesis of the pelvis depends largely on the precision of the osteotomy. An image guided navigation system could be helpful at this stage. So far, however, achieving a proper fit with a custom-made prosthesis has not proven to be a good mid- or long-term solution Citation[8].
In this report, we have presented a gender-specific pelvis SSM based on one of the largest training sets used to date. During the development of our model we observed that the quality of the pelvic reconstruction based on the SSM increased with the number of pelvises investigated in the training set and with the sub-division of the pelvises into anatomical subgroups (by gender). In the future, we plan to increase the numbers in the gender-specific training sets and also to account for other factors such as age and body mass index. It would also be very desirable to incorporate into our training sets pelvises of the younger population. The low reconstruction quality in the sacroiliac area reveals the deficiency of our approach for reconstruction of bony regions with high shape variability, anatomical shape variants, and highly individual but normal anatomy.
We conclude that the eight cases presented demonstrate the suitability of the SSM-based approach as a method for planning pelvic reconstruction and designing patient-specific artificial implants. This investigation showed that the SSM-based reconstruction method reconstructs the defect at the same clinically acceptable accuracy level (the surface deviation error being in the range of 1 mm) as the mirroring method. This proves the hypothesis that the presented method is a valuable tool for the planning of reconstructive surgery and the design of implants. Furthermore, we have shown that, with a reasonable number of datasets, we were able to create a clinically applicable gender-specific SSM that provided a stable basis for the planning of patient-specific pelvic reconstructions and implant design.
Acknowledgments
This research has been supported by the Department of Surgery at the University Hospital Basel and the ENDO-Stiftung, Hamburg, Germany. We also thank Dr. Stephanie Korn for her support with the statistical analysis and the radiologists Dr. Rolf Huegli (Bruderholz Hospital) and Dr. Thorsten Wischer (Merian-Iselin Hospital) for supporting the CT data collection.
Declaration of interest: The authors report no declaration of interest.
References
- Malawer M, Sugarbaker P. Musculoskeletal Cancer Surgery: Treatment of Sarcomas and Allied Diseases. Kluwer Academic Publishers, DordrechtThe Netherlands 2001
- Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am 1978; 60: 731–746
- Krieg AH, Mani M, Speth BM, Stalley PD. Extracorporeal irradiation for pelvic reconstruction in Ewing's sarcoma. J Bone Joint Surg Br 2009; 91: 395–400
- Delloye C, Banse X, Brichard B, Docquier PL, Cornu O. Pelvic reconstruction with a structural pelvic allograft after resection of a malignant bone tumor. J Bone Joint Surg Am 2007; 89: 579–587
- Kreutz M, Sendhoff B, Igel C. EALib: A C++ class library for evolutionary algorithms. Institute für Neuroinformatik, Ruhr-Universität Bochum, Bochum 2008
- Verma NN, Kuo KN, Gitelis S. Acetabular osteoarticular allograft after Ewing's sarcoma resection. Clin Orthop Relat Res 2004, 419: 149–154
- Aljassir F, Beadel GP, Turcotte RE, Griffin AM, Bell RS, Wunder JS, Isler MH. Outcome after pelvic sarcoma resection reconstructed with saddle prosthesis. Clin Orthop Relat Res 2005, 438: 36–41
- Bastian L, Hüfner T, Mossinger E, Geerling J, Goesling T, Busche M, Kendoff D, Bading S, Rosenthal H, Krettek C. [Integration of modern technologies in therapy of sarcomas of the pelvis. Computer-assisted hemipelvectomy and implantation of a “custom-made” Bonit gentamycin coated partial pelvic prosthesis] [In German]. Unfallchirurg 2003; 106: 956–962
- Falkinstein Y, Ahlmann ER, Menendez LR. Reconstruction of type II pelvic resection with a new peri-acetabular reconstruction endoprosthesis. J Bone Joint Surg Br 2008; 90: 371–376
- Gebert C, Gosheger G, Winkelmann W. Hip transposition as a universal surgical procedure for periacetabular tumors of the pelvis. J Surg Oncol 2009; 99: 169–172
- Ozaki T, Hillmann A, Bettin D, Wuisman P, Winkelmann W. High complication rates with pelvic allografts. Experience of 22 sarcoma resections. Acta Orthop Scand 1996; 67: 333–338
- Ozaki T, Hoffmann C, Hillmann A, Gosheger G, Lindner N, Winkelmann W. Implantation of hemipelvic prosthesis after resection of sarcoma. Clin Orthop Relat Res 2002, 396: 197–205
- Krol Z, Chapuis J, Schwenzer-Zimmerer K, Langlotz F, Zeilhofer H-F. Preoperative planning and intraoperative navigation in the reconstructive craniofacial surgery. J Med Informatics Technol 2005; 9: 83–89
- Paul L, Docquier P-L, Cartiaux O, Cornu O, Delloye C, Banse X. Inaccuracy in selection of massive bone allograft using template comparison method. Cell Tissue Bank 2008; 9: 83–90
- Paul L, Docquier P-L, Cartiaux O, Cornu O, Delloye C, Banse X. Selection of massive bone allografts using shape-matching 3-dimensional registration. Acta Orthop 2010; 81: 250–255
- Cootes TF, Taylor CJ, Cooper DH, Graham J. Active shape models – their training and application. Computer Vision and Image Understanding 1995; 61: 38–59
- Heimann T, Meinzer H-P. Statistical shape models for 3D medical image segmentation: A review. Med Image Anal 2009; 13: 543–563
- Lamecker H, Seebass M, Hege HC, Deuflhard P, A 3D statistical shape model of the pelvic bone for segmentation. In: Fitzpatrick JM, Sonka M, editors. Proceedings of SPIE Medical Imaging 2004: Image Processing, San Diego, CA, February 2004. Proc SPIE 2004;5370:1341–1351
- Meller S, Kalender WA, Building a statistical shape model of the pelvis. In: Lemke HU, Vannier MW, Inamura K, Farman AG, Doi K, Reiber JHC, editors. Proceedings of the 18th International Congress and Exhibition on Computer Assisted Radiology and Surgery (CARS 2004), Chicago, IL, June 2004. Amsterdam: Elsevier; 2004. pp 561–566
- Thompson S, Penney G, Buie D, Dasgupta P, Hawkes D, Use of a CT statistical deformation model for multi-modal pelvic bone segmentation. In: Reinhardt JM, Pluim JPW, editors. Proceedings of SPIE Medical Imaging 2008: Image Processing, San Diego, CA, February 2008. Proc SPIE 2008;6914:69141O
- Chan CSK, Barratt DC, Edwards PJ, Penney GP, Slomczykowski M, Carter TJ, Hawkes DJ, Cadaver validation of the use of ultrasound for 3D model instantiation of bony anatomy in image guided orthopaedic surgery. In: Barillot C, Haynor DR, Hellier P, editors. Proceedings of the 7th International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI 2004), Saint-Malo, France, September 2004. Part II. Lecture Notes in Computer Science 3217. Berlin: Springer; 2004. pp 397–404
- Seim H, Kainmueller D, Heller M, Lamecker H, Zachow S, Hege HC, Automatic segmentation of the pelvic bones from CT data based on a statistical shape model. In: Proceedings of the Eurographics Workshop on Visual Computing for Biomedicine (VCBM), Delft, The Netherlands, October 2008. pp 93–100
- Skadlubowicz P, Krol Z, Wrobel Z, Hefti F, Krieg A. Using of statistical shape models for pelvis reconstruction in the oncologic surgery. J Med Informatics Technol 2009; 13: 151–156
- Skadlubowicz P, Krol Z, Wrobel Z, Hefti F, Krieg A. Reconstruction of the pelvic region based on the statistical shape modeling. Advances in Intelligent and Soft Computing 2010; 69: 165–173
- Skadlubowicz P, Krol Z, Wrobel Z, Hefti F, Krieg A. Biometrical approach in the pelvis surgical reconstructive treatment. Int J Biometrics 2011; 3: 76–84
- Withey DJ, Koles ZJ, Medical image segmentation: methods and software. In: Proceedings of the 2007 Joint Meeting of the 6th International Symposium on Noninvasive Functional Source Imaging of the Brain and Heart and the International Conference on Functional Biomedical Imaging, Hangzhou, China, October 2007. pp 143–146
- Dunteman GH. Principal Components Analysis. Sage Publications, Newbury Park, CA 1989
- Jolliffe I. Principal Component Analysis. Springer, New York 2002
- Lorensen WE, Cline HE. Marching Cubes: A high resolution 3D surface construction algorithm. SIGGRAPH Comput Graph 1987; 21: 163–169
- Hsieh J. Computed Tomography: Principles, Design, Artifacts and Recent Advances. SPIE Press, Washington, DC 2003
- Raja V, Fernandes K. Reverse Engineering: An Industrial Perspective. Springer Verlag, London 2008
- Bookstein FL. Principal Warps - thin-plate splines and the decomposition of deformations. IEEE Trans Pattern Anal 1989; 11: 567–585
- Schroeder W, Martin K, Lorensen B, Avila L, Avila R, Law C. The Visualization Toolkit. Prentice Hall PTR, Upper Saddle River, NJ 1998
- Davies RH, Cootes TF, Twining CJ, Taylor CJ, An information theoretic approach to statistical shape modelling. In: Cootes TF, Taylor CJ, editors. Proceedings of the British Machine Vision Conference (BMVC 2001), Manchester, UK, September 2001. British Machine Vision Association; 2001. pp 3–11
- Edwards GJ, Cootes TF, Taylor CJ, Advances in active appearance models. In: Proceedings of the IEEE International Conference on Computer Vision (ICCV), Kerkyra, Corfu, September 1999. IEEE Computer Society; 1999. pp 137–142
- McInerney T, Terzopoulos D. Deformable models in medical image analysis: A survey. Med Image Anal 1996; 1: 91–108
- Osyczka A. Evolutionary Algorithms for Single and Multicriteria Design Optimization. Physica-Verlag, New York 2002
- Kramer O. Self-Adaptive Heuristics for Evolutionary Computation. Springer-Verlag, Berlin 2008
- Borgefors G. Hierarchical chamfer matching - a parametric edge matching algorithm. IEEE Trans Pattern Anal 1988; 10: 849–865
- Besl PJ, McKay ND. A method for registration of 3D shapes. IEEE Trans Pattern Anal 1992; 14: 239–256
- Heitz G, Rohlfing T, Maurer CR, Jr. Statistical shape model generation using nonrigid deformation of a template mesh. In: Fitzpatrick JM, Reinhardt JM, editors. Proceedings of SPIE Medical Imaging 2005: Image Processing, San Diego, CA, February 2005. Proc SPIE 2004;5747:1411–1421
- Zachow S, Lamecker H, Elsholtz B, Stiller M, Reconstruction of mandibular dysplasia using a statistical 3D shape model. In: Lemke HU, Inamura K, Doi K, Vannier MW, Farman AG, editors. Proceedings of the 19th International Congress and Exhibition on Computer Assisted Radiology and Surgery (CARS 2005), Berlin, Germany, June 2005. Amsterdam: Elsevier; 2005. pp 1238–1243
- Barratt DC, Chan CSK, Edwards PJ, Penney GP, Slomczykowski M, Carter TJ, Hawkes DJ. Instantiation and registration of statistical shape models of the femur and pelvis using 3D ultrasound imaging. Med Image Anal 2008; 12: 358–374
- Dworzak J, Lamecker H, von Berg J, Klinder T, Lorenz C, Kainmüller D, Seim H, Hege HC, Zachow S. 3D reconstruction of the human rib cage from 2D projection images using a statistical shape model. Int J Comput Assist Radiol Surg 2010; 5: 111–124
- Prassopoulos PK, Faflia CP, Voloudaki AE, Gourtsoyiannis NC. Sacroiliac joints: Anatomical variants on CT. J Comput Assist Tomogr 1999; 23: 323–327
- Badii M, Shin S, Torreggiani WC, Jankovic B, Gustafson P, Munk PL, Esdaile JM. Pelvic bone asymmetry in 323 study participants receiving abdominal CT scans. Spine (Phila Pa 1976) 2003; 28: 1335–1339
- Cooperstein R, Lew M. The relationship between pelvic torsion and anatomical leg length inequality: A review of the literature. J Chiropr Med 2009; 8: 107–118
- Cummings G, Scholz JP, Barnes K. The effect of imposed leg length difference on pelvic bone symmetry. Spine (Phila Pa 1976) 1993; 18: 368–373
- Gnat R, Saulicz E, Biały M, Kłaptocz P. Does pelvic asymmetry always mean pathology? Analysis of mechanical factors leading to the asymmetry. J Human Kinetics 2009; 21: 23–32
- Wong KC, Kumta SM, Chiu KH, Cheung KW, Leung KS, Unwin P, Wong MCM. Computer assisted pelvic tumor resection and reconstruction with a custom-made prosthesis using an innovative adaptation and its validation. Comput Aided Surg 2007; 12: 225–232