Abstract
Introduction: Individual planning of complex maxillofacial corrections may require 3D models which can be manufactured based on DICOM datasets. The gold standard for image acquisition is still high-resolution multi-slice computed tomography (MSCT). However, appropriate datasets for model fabrication can be acquired by modern Cone-Beam CT (CBCT) devices that have been developed specifically for maxillofacial imaging. The clinical utility of individual models fabricated on the basis of CBCT datasets was assessed.
Methods: In five patients affected by different deficiencies of the maxillofacial skeleton, preoperative imaging was performed with ILUMA CBCT. Segmentation of hard tissues was performed manually by thresholding. Corresponding STL datasets were created and exported to an industrial service provider (Alphaform, Munich, Germany) specializing in rapid prototyping, and 3D models were fabricated by the selective laser sintering (SLS) technique. For variance analysis, landmark measurements were performed on both virtual and 3D models. Subsequently, maxillofacial surgery was performed according to the model-based planning.
Results: All CBCT-based DICOM datasets could be used for individual model fabrication. Detailed reproduction of individual anatomy was achieved and a topographic survey showed no relevant aberrance between the virtual and real models. The CBCT-based 3D models were therefore used for planning and transfer of different maxillofacial procedures.
Conclusions: CBCT-based datasets can be used for the fabrication of surgical 3D models if the correct threshold is set. Preoperative workflow and patient comfort is improved in terms of the fast-track concept by using this “in-house” imaging technique.
Introduction
For approximately 25 years, individual three-dimensional (3D) models have been used for the planning of craniomaxillofacial corrections [Citation1, Citation2]. Technologies for image acquisition and data processing have evolved rapidly during this time, and computer assisted surgery is now well established in this field.
The gold standard for the fabrication of individual 3D models remains DICOM (Digital Imaging and Communication in Medicine) datasets that have been acquired by conventional high-resolution multi-slice computed tomography (MSCT) [Citation3]. Modern Cone-Beam CT (CBCT) scanners, which have now been used routinely in maxillofacial imaging for approximately 15 years [Citation4–6], generate DICOM datasets which can also be used for model production [Citation7]. Analysis of the accuracy of CBCT-based models has suggested that they are suitable for clinical use [Citation8]. However, the utility of such models in complex maxillofacial surgery should be assessed for different clinical applications.
Materials and methods
In 2009 Lambrecht et al. [Citation7] published their basic workflow for the production of CBCT-based 3D models, which was modified slightly in our study.
In five patients affected by different deficiencies of the maxillofacial skeleton, preoperative imaging was performed using an ILUMA® CBCT Scanner (ILUMA, IMTEC Europe, Oberursel, Germany) using the Large Field of View (LFOV) modality, which allows for a cylindrical volume of reconstruction of up to 21.1 cm × 14.2 cm. Scans were performed at standard settings for adult patients (enhanced mode, 3.8 mA, 40-second scanning time). The corresponding software for treatment planning and simulation, ILUMAVisionDental (V.2.1.2.8988), was used to render virtual models. These datasets were subsequently exported in DICOM format to our in-house-developed planning software, RapidSplint (Berliner Zentrum für Mechatronische Medizintechnik, Berlin, Germany [Citation9]). The segmentation of hard tissues was performed manually by the implemented threshold function, then the segmented datasets were piped through the implemented Marching Cubes algorithm in order to create the corresponding STL (Standard Triangular Language format/Surface Tessellation Language) datasets, which are required for manufacture of a model by rapid-prototyping technology.
Conversion and rendering of the datasets was performed and virtual models were created by RapidSplint and then compared to the initial 3D reconstructions of the ILUMAVision software. After the rendered datasets were approved, they were transferred to a professional service provider specializing in rapid-prototyping technologies (Alphaform, Feldkirchen, Germany, www.alphaform.de) by web-based file transfer protocol. Before fabrication of the models by the selective laser sintering (SLS) technique, another rendering was performed, and the models were cross-checked and finally approved by the surgeon. The models were delivered in a few days, and cost between 400 and 700 € each, depending on the size of the individual model. Based on this sequence, the additional time required for generating a patient-specific model must be calculated as 2–3 hours (for thresholding, data processing, crosschecking, and administration).
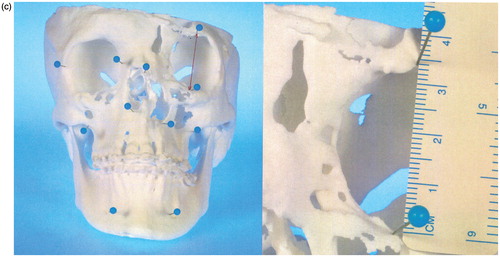
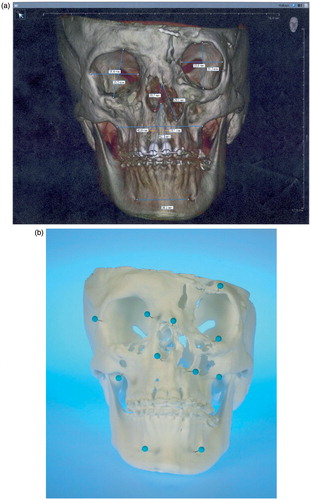
Before surgery, a topographic survey of the models was performed manually by landmark measurement and compared to the initial virtual models (ILUMAVision) in order to check for variations. Corresponding anatomic landmarks within the same plane were determined and multiple linear measurements were performed by the engineer and surgeon together for each pair of models (i.e., the virtual model and the corresponding SLS model). For the virtual model measurements, the calibrated software tool ILUMAVisionDental was used. For the SLS model, measurements of corresponding landmarks were performed with a surgical ruler (). Subsequently, the individual maxillofacial surgery was planned and performed according to the model-based planning.
Figure 1. (a) Virtual model rendered by ILUMAVision software and (b) the corresponding SLS model. Individual anatomy is reproduced by SLS technology in detail (e.g., the left paramedian craniofacial cleft). A topographic survey by landmark measurements demonstrated variations between the models of less than 1 mm, which was assessed to be sufficient for surgical use. (c) Measurement of the left anterior orbital height on the virtual and SLS models.
Results
All CBCT-based DICOM datasets could be used for individual model production. Accurate reproduction of individual anatomy was achieved by manual segmentation of bony tissues. Segmentation of skeletal structures could be performed directly after data acquisition on a clinical workstation without any loss of time. Depending on the threshold setting, typical partial volume effects and artefacts arising from orthodontic arch bars and dental restorations were observed which did not differ from the artefacts that are encountered when using classic MSCT datasets. Morphology and anatomic details of the fabricated models showed very good coincidence with the virtual models (). Comparison of landmark measurements between the virtual and real models showed good accordance, with variance in landmark distances being less than 1 mm. Therefore, all the CBCT-based models were assessed as being sufficiently accurate and they were used for further planning and transfer of different maxillofacial procedures.
Case 1 ()
The first patient for whom CBCT data were used for model production was referred to the department for the correction of a severe skeletal class III malocclusion in an edentulous upper jaw. Even with the use of dental implants, there was no possibility of sufficient prosthodontic treatment without prior surgical correction of the jaw relation.
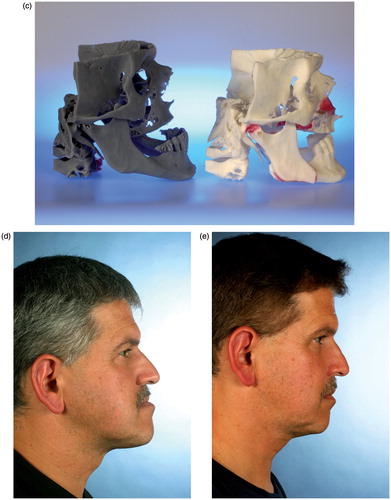
Figure 2. (a–c) CBCT-based models of the first clinical case, with a class III malocclusion and an edentulous maxilla (the models are available in different versions). A two-jaw model surgery was required for correct alignment of the alveolar processes prior to implant insertion. (d–e) The clinical situation before and after orthognathic correction supported by CBCT-based models.

Two CBCT-based models were manufactured by the company to demonstrate different types of manufacturing (). One of these models was used for model surgery (), which demonstrated clearly the need for a two-jaw correction in order to achieve proper alignment of the jaw relation (). The real surgery was then performed according to the treatment planning, and successful correction of the skeletal discrepancy was realized (). Prosthodontic rehabilitation is still ongoing at this time.
Case 2 ()
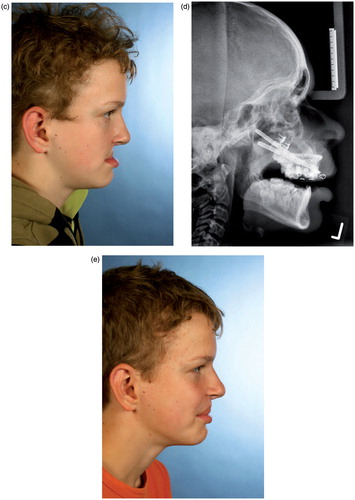
A twelve-year-old boy with a severe class III malocclusion due to a bilateral cleft lip and palate was referred to the department for surgical correction (). From the clinical situation, it was already clear that the discrepancy between the upper and lower jaw was too large to be corrected by a classic Le Fort I advancement, so maxillary distraction was intended. As the patient was an ardent soccer player, external distraction devices were not an option. Models were therefore produced in order to select appropriate distraction devices. To avoid dental interference which could block maxillary advancement during active distraction, an occlusal splint was also manufactured. Surgery and distraction were performed according to the CBCT model-based planning (). After removal of material, the distraction devices were mounted to the models and both the splint and the devices demonstrated an excellent fit with the surgical model (). Six months after consolidation there is an obvious improvement in the clinical situation ().
Figure 3. (a and b) CBCT-based models of a distraction case, showing the internal maxillary distraction device selected according to the model geometry, the additional acrylic splint to avoid occlusal interference, and the original devices mounted after removal of material. (c–e) Corresponding clinical pictures of the patient, who presented with a severe maxillary deficiency due to bilateral cleft: (c) The preoperative situation. (d) A cephalogram acquired during the active distraction phase. (e) The clinical situation after six months of consolidation.
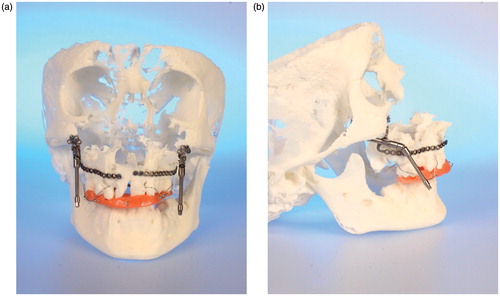
This workflow has also been applied in three other patients where therapy is still ongoing, with comparable advantages. In these patients, multiple surgeries will be necessary to improve the underlying skeletal pathologies. All of them had already undergone distraction procedures according to Ilizarov’s concept of gradual tissue expansion. Appropriate buried distraction devices were selected according to the specific CBCT-based model anatomy as proposed by Poukens et al. [Citation10]. So far, no obvious discrepancy between the model and real anatomy has been observed. Consequently, surgery was clearly supported by the CBCT models. One adult female patient affected by craniofacial cleft syndrome () underwent nasal distraction; an adult male patient affected by bilateral cleft lip and palate who lost the premaxilla due to precedent surgeries abroad underwent bilateral segmental maxillary distraction; and the third patient, a young man affected by severe mandibulomaxillary growth restriction due to post-traumatic unilateral TMJ ankylosis on the left side, had already undergone complex corrective surgery including arthrolysis and transpalatal distraction. In all five patient cases where CBCT-based 3D models were used, the models proved to be absolutely appropriate for surgical planning and transfer.
Discussion
The possibility of fabricating individual 3D models which clearly demonstrate the underlying skeletal pathology must certainly be ranked as representing substantial progress in the planning of corrective craniomaxillofacial surgery [Citation11]. Ongoing technical developments have contributed to the fact that contemporary surgery is computer assisted (CAS), and such development is likely to continue. User-friendly interfaces which shorten operational procedures certainly improve the acceptance and distribution of these techniques.
MSCT has been the gold standard for imaging of bony structures of the craniofacial region, and the DICOM format has contributed to the exchange of data files between different applications [Citation3]. Since its introduction to the field of maxillofacial surgery [Citation4, Citation5], CBCT has gained wide acceptance due to its inherent advantages compared to classic MSCT. These advantages generally include higher resolution of the images, lower radiation exposure for the patient, and the availability of additional diagnostic tools depending on the related software packages [Citation12].
Rapid-prototyping techniques are well established in biomedical engineering [Citation13], and there are several different techniques for creating 3D models based on individual datasets. Selective laser sintering (SLS) is a solid freeform fabrication technique that produces models through a selective solidification of a variety of fine powders in a layer-based methodology. This method generally provides a high level of accuracy [Citation14].
Detail reproduction and precision in 3D models depends on a variety of factors, key elements being the datasets that are used, as well as the method of technical production. In this study, segmentation of hard tissues was performed on an individual basis using the thresholding function of the ILUMAVision software. According to Loubele et al. [Citation15], CBCT scanners perform better for bony detail assessment compared to MSCT. After segmentation, these datasets were exported and used for further processing.
Kaim et al. [Citation16] found a high accuracy for craniofacial models that had been manufactured by SLS technology based on MSCT DICOM data when compared to the original CT data, and raised the question as to whether there might be alternative techniques involving less radiation exposure. In 2007 Kim et al. had already used CBCT data to replicate dental models in order to manufacture drilling guides for orthodontic screws [Citation17], and in 2008 Periago et al. concluded that CBCT datasets would be sufficiently accurate for clinical craniofacial analyses [Citation18]. According to the specifications of the Alphaform company, technical realization of the models has an accuracy/irregularity of ±0.25 mm and ±0.15% (www.alphaform.de). Landmark measurements showed a good correspondence between the virtual models and the SLS-fabricated models within this range. The models were therefore assessed as being suitable for surgical planning, and surgery was performed accordingly. In 2009 Lambrecht et al. [Citation7] published their workflow for 3D model fabrication based on CBCT datasets; however, the authors concluded that automated routine fabrication was too time-consuming and expensive [Citation7].
From the surgical point of view, there is a clear advantage in using CBCT datasets for surgical model production in selected cases: By using the workflow described in this article, we obtained highly precise models suitable for surgical planning and transfer, reduced the time and effort required for preoperative planning, and provided additional comfort for the patient. If CBCT devices are available within a maxillofacial unit there is no need for additional radiology services. Preoperative workflow and patient comfort was improved by using this “in-house” imaging technique, and the time and effort required for preoperative planning should not exceed the time for real surgery. In the presented cases, either complex or multi-step procedures were planned, so the effort seemed to be justified.
There are some drawbacks to this approach, however. Despite the fact that the ILUMA CBCT device currently offers the largest investigational volume compared to other CBCT scanners, the technique-related smaller scanning volumes, as compared to those in conventional MSCT, limit their use, especially with respect to craniofacial applications. Also, any disturbance during data acquisition (e.g., movements) will result in artefacts which cannot be corrected by manual thresholding, preventing the proper manufacture of a model. However, this can be assessed subsequent to data acquisition when the virtual models are checked. Whether there is really a reduction in radiation exposure for the patient, as compared to MSCT, could not be answered by this feasibility study.
Conclusion
According to this workflow, CBCT-acquired DICOM datasets can be used for the production of 3D models that are suitable for skeletal craniomaxillofacial corrections. Correct segmentation of skeletal structures and further data processing is supported by contemporary software solutions. From the surgical point of view, the main advantage of this workflow becomes apparent when datasets can be directly acquired and processed within the maxillofacial unit without the need for additional radiology services.
Declaration of interest
The authors report no declaration of interest.
References
- Brix F, Lambrecht JT. 1987. [Preparation of individual skull models based on computed tomographic information] [In German]. Fortschr Kiefer Gesichtschir 32:74–7
- Lambrecht JT, Brix F. 1990. Individual skull model fabrication for craniofacial surgery. Cleft Palate J 27(4):382–5
- Lambrecht J, editor. 1995. 3D-Modeling Technology in Oral and Maxillofacial Surgery. Quintessence Publishing
- Arai Y, Tammisalo E, Iwai K, Hashimoto K, Shinoda K. 1999. Development of a compact computed tomographic apparatus for dental use. Dentomaxillofac Radiol 28(4):245–8
- Mozzo P, Procacci C, Tacconi A, Martini PT, Andreis IA. 1998. A new volumetric CT machine for dental imaging based on the cone-beam technique: preliminary results. Eur Radiol 8(9):1558–64
- Keeve E, Hey J, Kusch J, Ritter L. 2008. Fundamentals of DVT technology. In: Zoeller J. Digital Volume Tomography in Dental, Oral and Maxillofacial Medicine. Berlin: Quintessenz. 3–22
- Lambrecht JT, Berndt DC, Schumacher R, Zehnder M. 2009. Generation of three-dimensional prototype models based on cone beam computed tomography. Int J Comput Assist Radiol Surg 4(2):175–80
- Liang X, Lambrichts I, Sun Y, Denis K, Hassan B, Li L, Pauwels R, Jacobs R. 2010. A comparative evaluation of Cone Beam Computed Tomography (CBCT) and Multi-Slice CT (MSCT). Part II: On 3D model accuracy. Eur J Radiol 75(2):270–4
- Liu W, Holzwarth F, Adolphs N, Hoffmeister B, Keeve E. 2013. A novel concept of intelligent parametric modeling of surgical splint based on clinic empirical assessment in orthognathic surgery. Presentation at the 27th International Congress and Exhibition on Computer Assisted Radiology and Surgery (CARS 2013), Heidelberg, Germany, June 2013
- Poukens J, Haex J, Riediger D. 2003. The use of rapid prototyping in the preoperative planning of distraction osteogenesis of the cranio-maxillofacial skeleton. Comput Aided Surg 8(3):146–154
- Sinn DP, Cillo JE Jr, Miles BA. 2006. Stereolithography for craniofacial surgery. J Craniofac Surg 17(5):869–75
- De Vos W, Casselman J, Swennen GR. 2009. Cone-beam computerized tomography (CBCT) imaging of the oral and maxillofacial region: a systematic review of the literature. Int J Oral Maxillofac Surg 38(6):609–25
- Esses SJ, Berman P, Bloom AI, Sosna J. 2011. Clinical applications of physical 3D models derived from MDCT data and created by rapid prototyping. AJR Am J Roentgenol 196(6):W683–W688
- Mazzoli A. 2013. Selective laser sintering in biomedical engineering. Med Biol Eng Comput 51(3):245–56
- Loubele M, Maes F, Jacobs R, van Steenberghe D, White SC, Suetens P. 2008. Comparative study of image quality for MSCT and CBCT scanners for dentomaxillofacial radiology applications. Radiat Prot Dosimetry 129(1–3):222–6
- Kaim AH, Kirsch EC, Alder P, Bucher P, Hammer B. 2009. [Preoperative accuracy of selective laser sintering (SLS) in craniofacial 3D modeling: comparison with patient CT data] [In German]. Rofo 181(7):644–51
- Kim SH, Choi YS, Hwang EH, Chung KR, Kook YA, Nelson G. 2007. Surgical positioning of orthodontic mini-implants with guides fabricated on models replicated with cone-beam computed tomography. Am J Orthod Dentofacial Orthop 131(4 Suppl):S82–9
- Periago DR, Scarfe WC, Moshiri M, Scheetz JP, Silveira AM, Farman AG. 2008. Linear accuracy and reliability of cone beam CT derived 3-dimensional images constructed using an orthodontic volumetric rendering program. Angle Orthod 78 (3):387–95