Abstract
This study was designed to investigate the association of the IL-8-251 T > A gene polymorphism with clinicopathological features and the prognostic role of the gene polymorphism in patients with gastric adenocarcinoma. The gene polymorphism was detected by the polymerase chain reaction–restriction fragment length polymorphism method, followed by univariate and multivariate analyses to elicit its prognostic role. The frequency of IL-8-251 A/A, A/T and T/T genotypes were 11.0% (23/210), 43.8% (92/210) and 45.2% (95/210), respectively. The IL-8-251 gene polymorphism was closely correlated with depth of invasion (p = 0.007), grade of differentiation (p = 0.002) and TNM stage (p = 0.009). A/A genotype carriers showed more frequency of serosa involvement, low grade of differentiation and advanced stage of gastric carcinoma. IL-8-251 T > A gene polymorphism have no significant correlation with other clinicopathological features. The 5-year overall survival of IL-8-251 A/A genotype and T allele carriers were 30.8% and 59.2%, respectively. There is a significant discrepancy among the different genotype carriers. Multivariate analysis with the Cox regression model revealed that the IL-8-251 A/A genotype is an independent prognostic indicator (HR = 2.285, 95% Confidence Interval = 1.06–4.93, p = 0.035). We conclude that the IL-8-251 A/A genotype may indicate a poor prognosis for gastric adenocarcinoma patients.
Introduction
Gastric carcinoma ranks as one of the most widespread malignancies worldwide with high fatality rates. Genetic polymorphisms conferring susceptibility to several types of cancer have been discovered (Jemal et al., Citation2011). Current staging systems and other clinicopathological indicators cannot precisely predict the prognosis of gastric carcinoma based on the high heterogeneity of this malignancy. Genetic markers of restriction fragment length polymorphism, microsatellite and single-nucleotide polymorphisms comprise the three phases of molecular genetics. Recently, a growing number of gene polymorphisms have been implicated with the development, progression and outcome of various types of cancer (Bell, Citation2011; Lakhani & Ashworth, Citation2001).
Interleukins (ILs) are key cytokines that have tumor-promoting effects, with single nucleotide polymorphisms in IL genes (including IL-1B, IL-1RN, IL-8, IL-10 and IL-16) which may significantly affect protein expression or function, and promote gastritis and cancer (Canas et al., Citation2009; Gao et al., Citation2009; Graziano et al., Citation2005; He et al., Citation2011; Lee et al., Citation2005; Li et al., Citation2007; Persson et al., Citation2009; Shin et al., Citation2008; Sugimoto et al., Citation2007; Wang et al., Citation2006; Xue et al., Citation2010; Yu et al., Citation2010; Yuzhalin, Citation2011; Zhou et al., Citation2011; Zhu et al., Citation2011). Some of these polymorphisms have been demonstrated to have the potential to predict prognosis. IL-8 has multiple functions which include stimulating endothelial cell division, inducing tumor cell migration, regulating neovascularization, increasing the metastatic potential of gastric carcinoma cells and thus, may have the potential to indicate the prognosis of gastric carcinoma patients (Kido et al., Citation2001; Konno et al., Citation2003; Lee et al., Citation2004; Macri et al., Citation2006). Investigation of IL-8 may provide more insight into the molecular mechanism of carcinogenesis, angiogenesis and the progression of gastric carcinoma.
There is a growing body of evidence that the polymorphism of IL-8-251 T > A significantly increases the risk of gastric carcinoma through an enhanced inflammatory process in Helicobacter pylori-infected patients. The IL-8-251 A/A genotype is associated with a higher risk of upper third location of stomach, diffuse type of Lauren’s classification, poorly differentiation and p53-mutated gastric carcinoma (Kang et al., Citation2009; Liu et al., Citation2010; Taguchi et al., Citation2005; Wang et al., Citation2010, Citation2012; Ye et al., Citation2009). Results of a meta-analysis of 42 case-control studies of the IL-8-251 T > A polymorphism and cancer risk showed that the carriers of the A allele had an elevated risk of developing breast cancer, gastric carcinoma and nasopharyngeal cancer (Canedo et al., Citation2008). Another meta-analysis of 2195 gastric carcinoma cases and 3505 controls also found that an IL-8 251 AA genotype was a risk factor for gastric carcinoma (Vinagre et al., Citation2011). Furthermore, studies showed that the association between the IL8-251 polymorphism and risk of gastric carcinoma is likely to be ethnicity specific and tends to be reproducible in populations of Asian origin (Freund et al., Citation2003; Ren et al., Citation2003; Song et al., Citation2010; Zhang et al., Citation2010). The prognostic value of IL-8-251 genotyping in gastric carcinoma cases has not been fully investigated.
We recruited 210 gastric carcinoma patients, collected metastasis-free lymph node tissue samples, detected the IL-8-251 T > A polymorphism and analyzed the correlation of the potential molecular marker with clinicopathological characteristics and patient prognosis, aiming to explore the potential of the polymorphism as a prognostic indicator.
Materials and methods
The genotyping had been described in the supplemental files.
Patients and tissue specimens
Between January 1999 and December 2000, patients who underwent clinical surgery for gastric adenocarcinoma at the Peking University School of Oncology were enrolled in this study. Patients with inadequate histological specimens or missing clinical information were excluded. A total of 210 consecutive patients were enrolled. Lymph node tissue samples pathologically confirming the lack of metastasis and suitable for DNA extraction were collected. Patients did not receive neo-adjuvant chemo-radiation. This study was approved by the ethics and research committee of the Peking University School of Oncology. Tumor staging was based on the 2002 sixth edition of AJCC/UICC TNM staging criteria for gastric carcinoma. All patients were followed up at regular intervals of 6 months after surgery with a minimum of 5 years of follow-up. Tumor recurrence was clinically defined as the reappearance of tumor after curative surgery. The overall survival time was calculated from the date of surgery to the last visit or death.
Statistical analysis
All statistical analyses were done using SPSS 10.0 software (SPSS Inc., Chicago, IL). The correlation analysis between the IL-8-251 T > A polymorphism and clinicopathologic characteristics was done using Pearson’s χ2 test. Cumulative survival probability as a function of time was calculated by the Kaplan–Meier method and the statistical significance of the difference between survival curves was estimated by the log-rank test. Two-sided t-test p values <0.05 were considered as statistically significant. Characteristics significantly or marginally associated with overall survival in the univariate analysis were included in the multivariate analysis. Multivariate survival analysis was performed with the Cox proportional hazards model by entering the IL-8-251 T > A genotype and established clinical and histopathologic features.
Results
Patient characteristics
There were 142 male and 68 female patients, and the median age was 56 years (range, 25–78 years). Thirty-four patients were classified as stage I, 52 as stage II, 64 as stage III and 60 as stage IV. A total of 133 cases were poorly differentiated, 43 cases were moderately differentiated and the remaining 34 cases were well differentiated. According to the Lauren classification, 70 cases were of the intestinal type, 97 were of the diffuse type and the remaining 43 were of mixed type. The follow-up period for survivors was at least 5 years, with a median of 34 months. The 5-year overall survival rate was 43.7% in the entire cohort of patient cases with 92.7% in stage I, 75.2% in stage II, 34.3% in stage III and 13.5% in stage IV patients.
The association between IL-8-251 polymorphic variants and clinicopathologic characteristics
Eleven percent (23 of 210) of the patients were homozygous for the A/A variant, 43.8% (92 of 210) were heterozygous for A/T, and 45.2% (95 of 210) of patients were homozygous for T/T variant. The association between the polymorphic variants and the clinicopathologic characteristics was carefully analyzed and is shown in . The IL-8-251 genotype was significantly correlated with depth of invasion (p = 0.007), differentiation (p = 0.002) and TNM stage (p = 0.009). Patients with A/A genotype were more likely to have serosa involvement, poor differentiation and more advanced stage.
Table 1. Association between IL-8-251 genotype and clinicopathologic factors.
The association between IL-8-251 polymorphic variants and patient prognosis
Univariate survival analysis showed that the 5-year overall survival rate of patients with the IL-8-251 A/T genotype was 30.8%, whereas patients with the A/T or T/T genotype were 59.2%, which is a statistically significant difference (p = 0.037, ). Patients with an A/A genotype had a poorer prognosis than those with A/T or T/T genotypes. Multivariate analysis adjusting for tumor size, lymphovascular invasion, Lauren classification, TNM stage and grade of differentiation identifies the IL-8-251 A/A genotype (RR = 2.285, 95% Confidence Interval = 1.06–4.93, p = 0.035, ) as a significant prognostic indicator.
Figure 1. Overall survival curves according to genotype of the IL-8-251 polymorphism in gastric carcinoma patients. Significant differences were observed between the A/A genotype and A/T & T/T genotype groups.
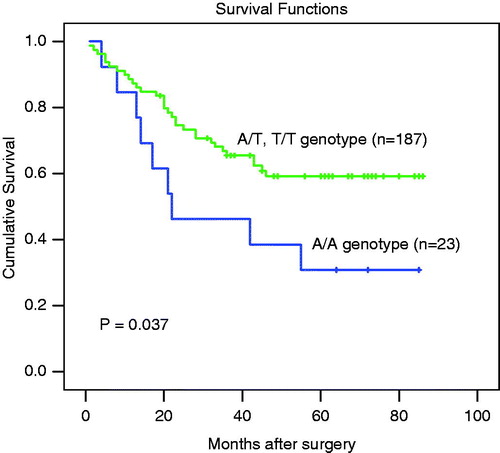
Table 2. Multivariate analysis of the prognostic impact of the IL-8-251 T > A polymorphism by the Cox proportional hazard model with a backward stepwise procedure.
Discussion
In this retrospective study, we detected the IL-8-251 T > A polymorphism in gastric carcinoma and found that an IL-8-251 A/A genotype closely correlated with depth of invasion, poorly differentiation and more advanced stage of gastric carcinoma. Patients with an A/A genotype are more likely to have serosa involvement, a low grade of differentiation and advanced stage of gastric carcinoma when compared to T allele carriers. Univariate and multivariate survival analyses revealed that the IL-8-251 T > A polymorphism was a significant independent prognostic indicator for overall survival in patients with gastric carcinoma. Patients with an IL-8-251 A/A genotype are more likely to have a decreased overall survival rate than patients with the T allele. Our data strongly support that the IL-8-251 T > A polymorphism has the potential to be used for prognostic predictions in patients with gastric carcinoma. IL-8-251 A/A genotype may not only serve as an indicator for the susceptibility to developing gastric carcinoma, but can also serve as a valuable prognostic indicator.
IL-8 is a multi-functional cytokine that can stimulate endothelial cell division and is a potent angiogenic factor, which directly correlates with the vascularity of human gastric carcinomas. IL-8 produced by tumor cells may regulate neovascularization and hence, the growth and spread of human gastric carcinoma (Kitadai et al., Citation1998). IL-8-transfected cells produced rapidly growing, highly vascular neoplasms (Kitadai et al., Citation1999). In H. pylori-infected patients, IL-8 is an important mediator of the inflammatory response that increases mucosal injury. A cDNA microarray study using gastric carcinoma cell lines (MKN-1 and TMK-1) co-cultured with H. pylori showed increased mRNA expression of IL-8 (Maeda et al., Citation2001). IL-8 might act as a diagnostic marker in patients with gastric carcinoma (Lee et al., Citation2004; Macri et al., Citation2006). IL-8 protein expression level is an independent and important prognostic factor in human gastric carcinomas (Kido et al., Citation2001). Measurement of circulating IL-8 levels in the serum of gastric carcinoma patients is a valuable indicator of risk for recurrent disease and may reflect IL-8 production mainly by the primary lesion (Konno et al., Citation2003). The prognostic role of IL-8 expression in tumor tissue, serum or urine has been extensively explored in multiple types of malignancies (hepatic cancer, breast cancer, ovarian cancer, colorectal cancer, bladder cancer, non-Hodgkin's lymphoma, prostate cancer, melanoma and neuroendocrine carcinomas); patients with high IL-8 expression have poorer prognosis than those with low expression (Ahmed et al., Citation2006; Benoy et al., Citation2004; Caruso et al., Citation2008; Derin et al., Citation2007; Freund et al., Citation2003; Kassim et al., Citation2004; Kocak et al., Citation2004; Lee et al., Citation2008; Merritt et al., Citation2008; Nastase et al., Citation2011; Ning et al., Citation2011; Pavel et al., Citation2005; Ren et al., Citation2003; Sadlecki et al., Citation2011; Terada et al., Citation2005; Zhang et al., Citation2011).
Studies have revealed that the IL-8-251 T > A functional polymorphism is associated with an increase in IL-8 synthesis by gastric epithelial cells, more severe neutrophils infiltration in epithelium and an increased risk of developing atrophic gastritis and gastric carcinoma (Canedo et al., Citation2008; Kang et al., Citation2009; Liu et al., Citation2010; Lu et al., Citation2005; Ohyauchi et al., Citation2005; Song et al., Citation2010; Taguchi et al., Citation2005; Vinagre et al., Citation2011; Wang et al., Citation2010, Citation2012; Ye et al., Citation2009; Zhang et al., Citation2010). The IL-8-251 A allele confers greater transcript activity than the T allele. The DNA sequence in the region around the IL-8-251 A allele may produce a potential binding site for C/EBP, and induce IL-8 expression through the nickel subsulfide-dependent pathway (Donn et al., Citation2002). Previous studies have explored the prognostic role of IL-8-251 T > A polymorphism in several other types of malignancies. A previous study showed that the IL-8-251 A/A homozygous genotype is strongly correlated with the aggressive phenotype of breast cancer, with the A allele significantly associated with decreased overall and disease-free survival (Smith et al., Citation2004; Snoussi et al., Citation2006). The IL-8 T-251 A allele was shown to be associated with shorter time to tumor recurrence, indicating that the analysis of angiogenesis-related gene IL-8 polymorphisms may help to identify a subgroup of patients at high risk for tumor recurrence (Lurje et al., Citation2008). Another previous study showed that the IL-8-251 T > A polymorphism might serve as a molecular predictor of response to bevacizumab-based therapy and determining the clinical outcome (Schultheis et al., Citation2008). Lurje et al. (Citation2010) demonstrated that the IL-8-251 T > A polymorphism was a significant prognosticator of the time to recurrence and overall survival in patients with gastric carcinoma. These results corroborate the findings in our study, as patients with the IL-8-251 A/A genotype had a higher likelihood to have more phenotypically aggressive and advanced stage tumors than patients with an A/T or T/T genotype, and as such, also exhibited a decreased overall survival.
The studies exploring the IL-8-251 T > A polymorphism primarily used fresh blood samples. Archival formalin-fixed paraffin-embedded tumor tissue may serve as a good resource for the exploration of new prognostic factors in a retrospective study. Recent studies have found that the fixation process does not seem to affect genotype detection. The genotyping results based on paraffin-embedded tissue DNA and peripheral blood DNA are highly consistent (Rae et al., Citation2003). Non-buffered, formalin-fixed paraffin-embedded gastric tissue from patients with gastric carcinoma serves as a valuable source of DNA, suitable for large-scale investigation. We selected lymph node tissue from patients with integral pathological examination and follow-up information to detect the polymorphism of IL-8-251; the lymph nodes were histopathologically confirmed to be metastasis-free.
There are still some limitations in our study. Firstly, because our study is a hospital-based study, our cancer center did not have enough tissue from the healthy people as control, since it is easier to obtain from the general hospital. Secondly, the sample size of this study is relatively low. Finally, it is a retrospective study, warranting a large sample size prospective study evaluation.
In summary, our results demonstrate that the IL-8-251 T > A polymorphism is correlated with depth of invasion, differentiation and progression of gastric carcinoma. The IL-8-251 A/A genotype may provide valuable prognostic information in gastric adenocarcinoma patients. This study provides a good index to further investigate the polymorphisms of ILs in gastric carcinoma survival prognostication. It would greatly facilitate our clinical practice and research work if the detection of IL polymorphisms could provide prognostic information.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
This work was supported by grants from the Key Technologies Research and Development Program (2002BA711A06), the National Basic Research Priorities Program (973), the Ministry of Science and Technology of China (1998051203), and the Beijing Science and Technology Commission (H020920030390).
Supplementary Material
Download PDF (139 KB)References
- Ahmed OI, Adel AM, Diab DR, Gobran NS. (2006). Prognostic value of serum level of interleukin-6 and interleukin-8 in metastatic breast cancer patients. Egypt J Immunol 13:61–8
- Bell CG. (2011). Accessing and selecting genetic markers from available resources. Methods Mol Biol (Clifton, NJ) 760:1–17
- Benoy IH, Salgado R, Van Dam P, et al. (2004). Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res 10:7157–62
- Canas M, Moran Y, Rivero M, et al. (2009). Interleukin-1 genetic polymorphism: association with gastric cancer in the high-risk Central-Western population of Venezuela. Rev Med Chile 137:63
- Canedo P, Castanheira-Vale AJ, Lunet N, et al. (2008). The interleukin-8-251*T/*A polymorphism is not associated with risk for gastric carcinoma development in a Portuguese population. Eur J Cancer Prev 17:28–32
- Caruso DJ, Carmack AJ, Lokeshwar VB, et al. (2008). Osteopontin and interleukin-8 expression is independently associated with prostate cancer recurrence. Clin Cancer Res 14:4111–18
- Derin D, Soydinc HO, Guney N, et al. (2007). Serum IL-8 and IL-12 levels in breast cancer. Med Oncol 24:163–8
- Donn R, Alourfi Z, De Benedetti F, et al. (2002). Mutation screening of the macrophage migration inhibitory factor gene: positive association of a functional polymorphism of macrophage migration inhibitory factor with juvenile idiopathic arthritis. Arthritis Rheum 46:2402–9
- Freund A, Chauveau C, Brouillet JP, et al. (2003). IL-8 expression and its possible relationship with estrogen-receptor-negative status of breast cancer cells. Oncogene 22:256–5
- Gao LB, Rao L, Wang YY, et al. (2009). The association of interleukin-16 polymorphisms with IL-16 serum levels and risk of colorectal and gastric cancer. Carcinogenesis 30:295–9
- Graziano F, Ruzzo A, Santini D, et al. (2005). Prognostic role of interleukin-1β gene and interleukin-1 receptor antagonist gene polymorphisms in patients with advanced gastric cancer. J Clin Oncol 23:2339–45
- He BS, Pan YQ, Xu YF, et al. (2011). Polymorphisms in interleukin-1B (IL-1B) and interleukin 1 receptor antagonist (IL-1RN) genes associate with gastric cancer risk in the Chinese population. Dig Dis Sci 56:2017–23
- Jemal A, Bray F, Center MM, et al. (2011). Global cancer statistics. Cancer J Clin 61:69–90
- Kang JM, Kim N, Lee DH, et al. (2009). The effects of genetic polymorphisms of IL-6, IL-8, and IL-10 on Helicobacter pylori-induced gastroduodenal diseases in Korea. J Clin Gastroenterol 43:420–8
- Kassim SK, El-Salahy EM, Fayed ST, et al. (2004). Vascular endothelial growth factor and interleukin-8 are associated with poor prognosis in epithelial ovarian cancer patients. Clin Biochem 37:363–9
- Kido S, Kitadai Y, Hattori N, et al. (2001). Interleukin 8 and vascular endothelial growth factor – prognostic factors in human gastric carcinomas? Eur J Cancer 37:1482–7
- Kitadai Y, Haruma K, Sumii K, et al. (1998). Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am J Pathol 152:93–100
- Kitadai Y, Takahashi Y, Haruma K, et al. (1999). Transfection of interleukin-8 increases angiogenesis and tumorigenesis of human gastric carcinoma cells in nude mice. Br J Cancer 81:647–53
- Kocak H, Oner-Iyidogan Y, Kocak T, Oner P. (2004). Determination of diagnostic and prognostic values of urinary interleukin-8, tumor necrosis factor-alpha, and leukocyte arylsulfatase-A activity in patients with bladder cancer. Clin Biochem 37:673–8
- Konno H, Ohta M, Baba M, et al. (2003). The role of circulating IL-8 and VEGF protein in the progression of gastric cancer. Cancer Sci 94:735–40
- Lakhani SR, Ashworth A. (2001). Microarray and histopathological analysis of tumours: the future and the past? Nat Rev Cancer 1:151–7
- Lee HL, Eom HS, Yun T, et al. (2008). Serum and urine levels of interleukin-8 in patients with non-Hodgkin’s lymphoma. Cytokine 43:71–5
- Lee JY, Kim HY, Kim KH, et al. (2005). Association of polymorphism of IL-10 and TNF-A genes with gastric cancer in Korea. Cancer Lett 225:207–14
- Lee KH, Bae SH, Lee JL, et al. (2004). Relationship between urokinase-type plasminogen receptor, interleukin-8 gene expression and clinicopathological features in gastric cancer. Oncology 66:210–17
- Li C, Xia HHX, Xie W, et al. (2007). Association between interleukin-1 gene polymorphisms and Helicobacter pylori infection in gastric carcinogenesis in a Chinese population. J Gastroenterol Hepatol 22:234–9
- Liu L, Zhuang W, Wang C, et al. (2010). Interleukin-8-251 A/T gene polymorphism and gastric cancer susceptibility: a meta-analysis of epidemiological studies. Cytokine 50:328–34
- Lu W, Pan K, Zhang L, et al. (2005). Genetic polymorphisms of interleukin (IL)-1B, IL-1RN, IL-8, IL-10 and tumor necrosis factor α and risk of gastric cancer in a Chinese population. Carcinogenesis 26:631–6
- Lurje G, Husain H, Power DG, et al. (2010). Genetic variations in angiogenesis pathway genes associated with clinical outcome in localized gastric adenocarcinoma. Ann Oncol 21:78–86
- Lurje G, Zhang W, Schultheis AM, et al. (2008). Polymorphisms in VEGF and IL-8 predict tumor recurrence in stage III colon cancer. Ann Oncol 19:1734–41
- Macri A, Versaci A, Loddo S, et al. (2006). Serum levels of interleukin 1beta, interleukin 8 and tumour necrosis factor alpha as markers of gastric cancer. Biomarkers 11:184–93
- Maeda S, Otsuka M, Hirata Y, et al. (2001). cDNA microarray analysis of Helicobacter pylori-mediated alteration of gene expression in gastric cancer cells. Biochem Biophys Res Commun 284:443–9
- Merritt WM, Lin YG, Spannuth WA, et al. (2008). Effect of interleukin-8 gene silencing with liposome-encapsulated small interfering RNA on ovarian cancer cell growth. J Natl Cancer Inst 100:359–72
- Nastase A, Paslaru L, Niculescu AM, et al. (2011). Prognostic and predictive potential molecular biomarkers in colon cancer. Chirurgia 106:177–85
- Ning Y, Manegold PC, Hong YK, et al. (2011). Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer 128:2038–49
- Ohyauchi M, Imatani A, Yonechi M, et al. (2005). The polymorphism interleukin 8 -251 A/T influences the susceptibility of Helicobacter pylori related gastric diseases in the Japanese population. Gut 54:330–5
- Pavel ME, Hassler G, Baum U, et al. (2005). Circulating levels of angiogenic cytokines can predict tumour progression and prognosis in neuroendocrine carcinomas. Clin Endocrinol 62:434–43
- Persson C, Engstrand L, Nyrén O, et al. (2009). Interleukin 1-β gene polymorphisms and risk of gastric cancer in Sweden. Scand J Gastroenterol 44:339–45
- Rae JM, Cordero KE, Scheys JO, et al. (2003). Genotyping for polymorphic drug metabolizing enzymes from paraffin-embedded and immunohistochemically stained tumor samples. Pharmacogenetics 13:501–7
- Ren Y, Poon RT, Tsui HT, et al. (2003). Interleukin-8 serum levels in patients with hepatocellular carcinoma: correlations with clinicopathological features and prognosis. Clin Cancer Res 9:5996–6001
- Sadlecki P, Walentowicz-Sadlecka M, Szymanski W, Grabiec M. (2011). Comparison of VEGF, IL-8 and beta-FGF concentrations in the serum and ascites of patients with ovarian cancer. Ginekol Pol 82:498–502
- Schultheis AM, Lurje G, Rhodes KE, et al. (2008). Polymorphisms and clinical outcome in recurrent ovarian cancer treated with cyclophosphamide and bevacizumab. Clin Cancer Res 14:7554–63
- Shin WG, Jang JS, Kim HS, et al. (2008). Polymorphisms of interleukin-1 and interleukin-2 genes in patients with gastric cancer in Korea. J Gastroenterol Hepatol 23:1567–73
- Smith KC, Bateman AC, Fussell HM, Howell WM. (2004). Cytokine gene polymorphisms and breast cancer susceptibility and prognosis. Eur J Immunogenet 31:167–73
- Snoussi K, Mahfoudh W, Bouaouina N, et al. (2006). Genetic variation in IL-8 associated with increased risk and poor prognosis of breast carcinoma. Hum Immunol 67:13–21
- Song JH, Kim SG, Jung SA, et al. (2010). The interleukin-8-251 AA genotype is associated with angiogenesis in gastric carcinogenesis in Helicobacter pylori-infected Koreans. Cytokine 51:158–65
- Sugimoto M, Furuta T, Shirai N, et al. (2007). Effects of interleukin-10 gene polymorphism on the development of gastric cancer and peptic ulcer in Japanese subjects. J Gastroenterol Hepatol 22:1443–9
- Taguchi A, Ohmiya N, Shirai K, et al. (2005). Interleukin-8 promoter polymorphism increases the risk of atrophic gastritis and gastric cancer in Japan. Cancer Epidemiol Biomarkers Prev 14:2487–93
- Terada H, Urano T, Konno H. (2005). Association of interleukin-8 and plasminogen activator system in the progression of colorectal cancer. Eur Surg Res 37:166–72
- Vinagre RM, Corvelo TC, Arnaud VC, et al. (2011). Determination of strains of Helicobacter pylori and of polymorphism in the interleukin-8 gene in patients with stomach cancer. Arq Gastroenterol 48:46–51
- Wang J, Pan HF, Hu YT, et al. (2010). Polymorphism of IL-8 in 251 allele and gastric cancer susceptibility: a meta-analysis. Dig Dis Sci 55:1818–23
- Wang N, Zhou R, Wang C, et al. (2012). -251 T/A polymorphism of the interleukin-8 gene and cancer risk: a HuGE review and meta-analysis based on 42 case-control studies. Mol Biol Rep 39:2831–41
- Wang P, Xia HHX, Zhang JY, et al. (2006). Association of interleukin-1 gene polymorphisms with gastric cancer: A meta-analysis. Int J Cancer 120:552–62
- Xue H, Lin B, Ni P, et al. (2010). Interleukin-1B and interleukin-1 RN polymorphisms and gastric carcinoma risk: a meta-analysis. J Gastroenterol Hepatol 25:1604–17
- Ye BD, Kim SG, Park JH, et al. (2009). The interleukin-8-251 A allele is associated with increased risk of noncardia gastric adenocarcinoma in Helicobacter pylori-infected Koreans. J Clin Gastroenterol 43:233–9
- Yu J, Zeng Z, Wang S, et al. (2010). IL-1B-511 polymorphism is associated with increased risk of certain subtypes of gastric cancer in Chinese: a case-control study. Am J Gastroenterol 105:557–64
- Yuzhalin A. (2011). The role of interleukin DNA polymorphisms in gastric cancer. Human Immunol 72:1128–36
- Zhang H, Fu T, McGettigan S, et al. (2011). IL8 and cathepsin B as melanoma serum biomarkers. Int J Mol Sci 12:1505–18
- Zhang L, Du C, Guo X, et al. (2010). Interleukin-8-251A/T polymorphism and Helicobacter pylori infection influence risk for the development of gastric cardiac adenocarcinoma in a high-incidence area of China. Mol Biol Rep 37:3983–9
- Zhou Y, Hu W, Zhuang W, Wu X. (2011). Interleukin-10 -1082 promoter polymorphism and gastric cancer risk in a Chinese Han population. Mol Cell Biochem 347:89–93
- Zhu Y, Wang J, He Q, Zhang JQ. (2011). The association between interleukin-10-592 polymorphism and gastric cancer risk: a meta-analysis. Med Oncol 28:133–6
