Abstract
Background: There is paucity of information on mechanisms constituting adverse birth outcomes. We assessed here the relationship between vascular integrity and adverse birth effects.
Methods and results: Third trimester maternal plasma (n = 144) from the Maternal-Infant Research on Environmental Chemicals Study (MIREC) was analysed for vascular, inflammatory and oxidative stress markers by HPLC-fluorescence, protein array and EIA method. Analysis of the <25th and >75th percentile birth weight subgroups revealed markers associated with birth weight (ETs, MMP-9, VEGF, and 8-isoPGF-2α) and gestational age (ET-1, MMP-2, and VEGF).
Conclusions: Mechanistic insights into adverse birth outcome pathways can be achieved by integrating information on multiple biomarkers, physiology using systems biology approach.
Introduction
Perinatal health outcomes are considered important indicators of future child and adult health (Perera et al., Citation2007; Stillerman et al., Citation2008; Woodruff et al., Citation2009). Globally, adverse birth outcomes rank among the top 10 causes for disability-adjusted life years (Murray & Lopez, Citation1997; World Health Organization, Citation2012). In 2009–2010, the annual burden of adverse perinatal outcomes for Canada were 18 302 preterm births, 1167 infant deaths, 19 097 small-for-gestational age singleton infants, 1613 fetal deaths and 11 441 congenital anomalies (Public Health Agency of Canada, Citation2013).
Various factors including maternal nutrition, environmental contaminant exposures and socioeconomic factors have been associated with adverse perinatal outcomes (Abu-Saad & Fraser, Citation2010; Alsuwaida et al., Citation2011; Gelson & Johnson, Citation2010; Jolly et al., Citation2000; Mathews et al., Citation1999; Sharnkardass et al., Citation2014; Wigle et al., Citation2008). Very young and advanced maternal ages have been linked to poor pregnancy outcomes (Chantrapanichkul & Chawanpaiboon, Citation2013; Kenny et al., Citation2013). Also, maternal smoking is implicated in miscarriage, perinatal mortality, birth defects, low birth weight and premature births (Hackshaw et al., Citation2011; Howe et al., Citation2012; Stangl et al., Citation2008).
Two measures that are typically considered in assessment of the quality of an infant’s development are birth weight and gestational age at delivery. Low birth weight is defined as <2500 g (JAMA, Citation2002). Several chronic health consequences in adulthood such as hypertension, diabetes mellitus and obesity are associated with low birth weight, meanwhile high birth weight is associated with cancer (Curhan et al., Citation1996; Ross, Citation2006). Normal term pregnancy is expected to last between 37 and 41 completed weeks, while preterm birth is defined as live birth before 37 weeks (Centers for Disease Control and Prevention, Citation2009). Low birth weight can be due to preterm birth or intra uterine growth restriction (IUGR), or a combination of the two. The most common measure of IUGR is small-for-gestational age (SGA) generally defined as birth weight below the 10th percentile for gestational age for a reference population. Two proposed mechanistic pathways for these perinatal outcomes are oxidative stress and endothelial dysfunction.
Elevated circulating levels of vasoconstrictor peptide endothelin-1 and high blood pressure (BP) in pregnancy are related to IUGR and low birth weight (Arslan et al., Citation2004). Furthermore, increased risk of future cardiovascular disease was associated with maternal and neonatal complications in pregnancy (Stangl et al., Citation2008). Similarly, maternal hypertension is implicated in increased risk of adverse cardiovascular health in offspring, later in childhood (Lawlor et al., Citation2012).
Oxidative stress plays a role in maternal and foetal morbidity (Bell et al., Citation2007; Cindrova-Davies, Citation2009; Gitto et al., Citation2002; Tabacova et al., Citation1998; Triche & Hossain, Citation2007), in pre-eclampsia (Burton & Jauniaux, Citation2004) and in preterm delivery (Ferguson et al., Citation2015a,Citationb). For instance, environmental exposures to phthalates are associated with increased oxidative stress markers such as 8-hydroxy-2′-deoxyguanosine (8-OHdG) (Guo et al., Citation2014) and 8-isoprostane (8-iso-PGF-2α) (Ferguson et al., Citation2014, 2015a,Citationb; Watkins et al., Citation2015) in pregnant women. Similarly, in vitro exposure of placental cells to phthalates resulted in oxidative stress-related DNA damage (Tetz et al., Citation2013). Exposure to air pollutants, which can trigger oxidative stress, has been linked to adverse pregnancy outcomes (Olsson et al., Citation2013; CitationTabacova, 2000).
Although there have been a few reports on a limited number of maternal markers and their association with IUGR, a meta-analysis suggested the need for incorporation of biophysical and maternal clinical characteristics along with maternal biomarkers when testing for such associations in order to meet the requirements of a clinically useful predictive test (Conde-Agudelo et al., Citation2013). Identification of maternal biochemical pathways relevant to birth outcomes can shed light on intervention strategies to improve future child health.
The goal of this work was to gain insight into maternal vascular mechanisms during pregnancy that potentially influenced infant birth weight. For this purpose, we analysed the third trimester plasma of a subset of mothers from a mother-infant cohort (The Maternal-Infant Research on Environmental Chemicals – MIREC) study for molecular markers relevant to maternal vascular health including some that are considered as prognostic factors of cardiovascular diseases (e.g. endothelins).
Methods
Materials
Dulbecco’s phosphate-buffered saline (PBS, calcium and magnesium free), ethylenediaminetetraacetic acid (EDTA), diethylenetriaminepentaacetic acid (DETPA), phenylmethylsulfonyl fluoride (PMSF), trifluoroacetic acid (TFA), 3,4-dichloroisocoumarin, molecular weight cut-off filters (30, 50 and 100 kDa) and endothelins (BET-1, ET-1, ET-2 and ET-3) were purchased from Sigma (St. Louis, MO). Reagent-grade acetone, acetonitrile, ethyl acetate and methanol were from commercial suppliers. Butylated hydroxytoluene (BHT) was from United States Biochemical Corporation (Cleveland, OH). Deionzed water (DI water) was obtained from a super-Q plus high purity water system (Millipore, Bedford, MA). UHP-grade compressed nitrogen was supplied by Matheson Gas products (Whitby, ON, Canada). Amber glass vials and screw caps with septa were purchased from Chromatographic specialities Inc. (Brockville, ON, Canada). Antiprotease (Halt protease inhibitor) cocktail was obtained from ThermoFisher (Ottawa, ON, Canada). Polyclonal 8-iso-PGF-2α antibody was purchased from Oxford Biomedical Research (Oxford, MI). The EIA assay kit for free 8-isoPGF-2α analysis was from Cayman Chemical Company (Ann Arbor, MI). Multiplex kits were purchased from either Millipore (Billerica, MA) or BioRad (Mississauga, ON, Canada).
Maternal data and biospecimen collection
Third trimester maternal blood plasma were obtained from the Maternal–Infant Research on Environmental Chemicals (MIREC) Study cohort described by Arbuckle et al. (Citation2013). Plasma samples from a random subset (since these are from the first batch of plasma samples dispatched to our laboratory by the MIREC Study bio bank as they received from the different participating study sites across Canada) of subjects (n = 144) were analysed to gain preliminary information on maternal vascular mechanisms and their impact on birth outcomes. Maternal systolic and diastolic blood pressure values were measured during the third trimester clinic visit when the blood samples were collected. Infant birth weight and gestational age were extracted from the medical charts at delivery. Pre-pregnancy BMI was obtained by self-report in the first trimester questionnaire. Only singleton births were considered in this study.
Ethics
The details of the ethics review of the MIREC study are described by Arbuckle et al. (Citation2013). Briefly, the research protocol, questionnaires, consent forms and recruitment posters and pamphlets were reviewed and approved by human studies research ethics committees, including the Research Ethics Board at Health Canada and the ethics committee at the coordinating centre at St-Justine’s Hospital in Montreal, as well as more than 10 academic and hospital ethics committees across Canada. All participants signed informed consent forms.
Plasma sample preparation
Plasma samples were derived from the 3rd trimester maternal whole blood samples (n = 144) stabilized with preservatives (EDTA, PMSF) following a procedure described by Kumarathasan et al. (Citation2001). These plasma samples were treated with DETPA, BHT and antiprotease cocktail, vortexed, and were frozen for storage (Kumarathasan et al., Citation2001). Matching sets of aliquots of plasma were analysed for target biomarkers namely, circulating endothelins, vascular endothelial growth factor (VEGF) and other cardiovascular markers such as 8-iso-PGF-2α, matrix metalloproteinases (MMPs) and acute phase proteins [e.g., C-reactive protein (CRP), cellular adhesion molecules ICAM-1 and VCAM-1].
Circulating endothelin isoforms
This procedure was conducted as described by Kumarathasan et al. (Citation2001). Briefly, aliquots of the 3rd trimester maternal plasma samples (250 μL) were treated with 3,4-dichloroisocoumarin solution in isopropanol to prevent conversion of big ET-1 to ET-1 during sample processing. These samples were then de-proteinized with acidified acetone, followed by clean-up using molecular weight cut-off filters (30 kDa). Clarified samples were dried under a N2 flow, and were reconstituted in the mobile phase A (composition is given below), and were analysed by a reversed phase HPLC-Fluorescence system. Initial separation of endothelin isoforms (Big ET-1, ET-1, ET-2 and ET-3) were carried out on a LC-318 column (25 cm length, 4.6 mm id, 5 μm particle size; Supelco, Oakville, ON) by gradient elution using water-acetonitrile mobile phase (A-30% acetonitrile (aq); B-90% acetonitrile (aq)) with 0.19% of TFA used as the ion-pair reagent. Analytes were measured by fluorescence detection at excitation and emission wavelengths of 240 nm and 380 nm, respectively.
Affinity-based multiplex protein array analyses
Analysis of maternal plasma samples for target markers, namely vascular endothelial growth factor (VEGF-A), acute phase proteins C-reactive protein (CRP), soluble intracellular adhesion molecule (ICAM-1), soluble vascular cell adhesion molecule (VCAM-1) and matrix metalloproteinases (MMPs) were conducted by affinity-based multiplex protein array assays using Bio-Plex Pro Human panels (Biorad, Mississauga, ON, Canada) and Milliplex Map kits (Millipore, Bedford, MA). Briefly, plasma samples were incubated with capture antibody-coated magnetic beads, then washed and reacted with biotinylated-detection antibodies followed by incubation with streptavidin-phycoerythrin. The bead complex was washed and re-suspended in sheath fluid (Biorad, Mississauga, ON, Canada) and analysed using a Bioplex 100 instrument with Bioplex Manager 6.0 software (Biorad, Mississauga, ON, Canada).
Plasma 8-iso-PGF-2α
Aliquots of plasma (250 μL) samples were stabilised with DETPA and BHT to prevent any autoxidation during the 8-iso-PGF-2α analysis. These samples were then deproteinized, clarified with ethyl acetate and affinity purified by using a polyclonal 8-iso-PGF-2α antibody following the procedure described by Bielecki et al. (Citation2012). Purified plasma samples were then analysed for 8-iso-PGF-2α using the EIA kit from Cayman chemical (Ann Arbor, MI).
Statistical analyses
Initially, associations among maternal plasma biomarkers, blood pressure (BP), pre-pregnancy BMI, age and pregnancy outcomes (IBW, GA, IBW/GA ratio) were tested using Pearson Product Moment Correlation (SigmaStat v3.5, SPSS Inc., Chicago, IL) for all data (n = 144). Multivariate regression analyses were then performed by best subsets regression analyses (SigmaStat v3.5, SPSS Inc., Chicago, IL) to identify maternal factors that were influencing birth outcomes. In addition, polytomous logistic regression analyses were also performed using SAS EG, v4.2 (SAS institute Inc., Cary, NC) to test the sensitivity of the results to the choice of the model, and the final model was chosen based on AIC criteria (Akaike, Citation1974). The following independent variables were used in the multivariate regression analyses: Maternal pre-pregnancy BMI, age, systolic BP (SysBP), diastolic BP (DiaBP), and plasma BET-1, ET-1, ET-2, ET-3, 8-iso-PGF-2α (ISOP), CRP, ICAM-1, VCAM-1, MMP-1, MMP-2, MMP-7, MMP-9, MMP-10 and VEGF-A. These independent variables were chosen based on the Pearson Product Moment Correlation test results. In order to conduct best subsets regression (Neter et al., Citation1985) and polytomous logistic regression analyses, the following steps were taken. (A) Infant birth weight data were categorized into <25th percentile (Level 1), 25th–75th (Level 0) and >75th percentile (Level 2) (IBW-distribution), or (B) the birth weight data were categorized into Levels 1–3 (definition same as above) based on birth weight percentile for gestational age reference values reported on a larger Canadian population study (Kramer et al., Citation2001), taking the sex of the infant into consideration (IBW-GA distribution). Birth outcomes tested for with the categorized data in the IBW-distribution approach were infant birth weight (IBW), gestational age (GA) or IBW/GA ratio (an attempt to mathematically correct for GA by normalization), while with the categorized data from the IBW-GA distribution approach, the birth outcome tested was only IBW. Both regression analyses were conducted either on all categorized data (Levels 1–3), or on <25th percentile and 25th–75th percentile (Levels 1 and 0), or on >75th percentile and 25th–75th percentile (Levels 0 and 2) categorized data.
Protein interaction networks and biofunctions were identified using Ingenuity Pathway Analysis (IPA) (Ingenuity Systems, www.ingenuity.com) based on plasma protein marker changes in the <25th percentile and >75th percentile groups compared with the 25th–75th percentile group.
Results
Maternal characteristics namely age, body mass index, systolic and diastolic blood pressure and associated pregnancy outcomes are summarized in . The infants were 52% males and 48% females. Moreover, an overlay of the distribution curves for IBW, GA and IBW/GA ratio for the pregnancies assessed here (n = 144) versus the complete MIREC study population are presented in .
Figure 1. Distribution profiles for the n = 144 pregnancies. (A) Infant birth weight. (B) Gestational age. (C) Birth weight/gestational age. Black circles: subsample for current study (n = 144). Empty circles: Entire MIREC Cohort.
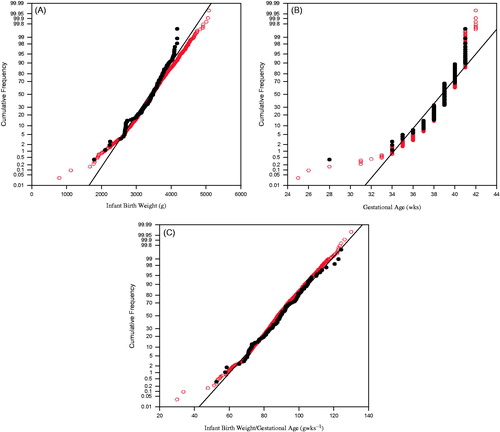
Table 1. Characteristics of the mothers and pregnancy outcomes.
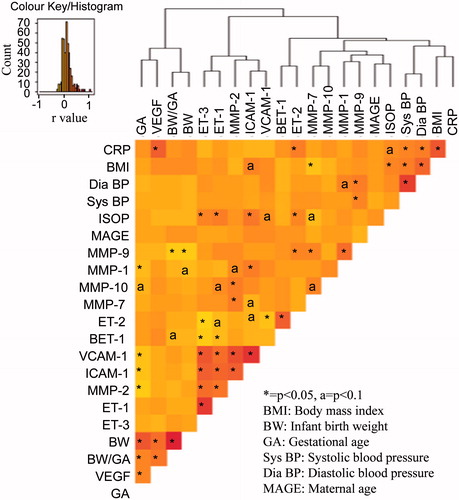
Correlations among the maternal biological markers, maternal physiological parameters and birth outcomes are illustrated by hierarchical clustering and heatmap for all data () and the corresponding raw data are provided in Supplemental . Significant (p < 0.05) positive associations were seen among markers, namely ET-1, ET-3, ICAM-1, VCAM-1, MMP-2 and thus were clustered together in the heatmap (). Maternal DiaBP, SysBP, BMI and CRP clustered together. There were positive correlations noted among GA, BW and maternal circulating VEGF.
Figure 2. Heatmap and hierarchical clustering of correlation analysis results for all data. Color key/histogram indicates the strength of correlation (r values). Darker (red) shade indicates positive correlation. Lighter (yellow) shade indicates negative correlation.
Best subsets regression (Neter et al., Citation1985) models revealed a combination of maternal factors influencing pregnancy outcomes for IBW and IBW-GA distributions (). The optimal regression model for the infant birth weight (IBW) outcome by use of IBW distribution data consisted of ICAM-1, VCAM-1, VEGF and ET-3. For GA as an outcome, the model contained MMP-2, VEGF, ICAM-1 and ET-1, and the optimal model for the IBW/GA ratio was composed of maternal plasma BET-1, MMP-2 and -9, VEGF and BMI.
Table 2. Maternal markers affecting different birth outcomes as determined by regression model analyses.
Meanwhile, the optimal model for infant birth weight outcome using the IBW-GA distribution data consisted of VCAM-1, ICAM-1, VEGF, BMI, DiaBP and ET-1. Moreover, the list of maternal markers that were related to the <25th percentile and >75th percentile groups (versus the 25th–75th percentile group) identified through multivariate regression analyses of the IBW and IBW-GA distribution data are also provided in . Both regression analyses yielded somewhat similar findings.
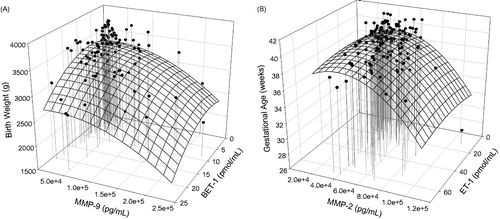
The commonality and differences in the nature of maternal markers constituting these regression models for the different IBW distributions are illustrated in . Similarly, the profiles of selected maternal markers of biochemical changes associated with infant birth weight and gestational age identified through these regression analyses are illustrated in .
Figure 3. Venn diagram of maternal factors dictating infant birth weight based on infant birth weight distribution data analysed by best subsets regression analyses. (A) All data. (B) <25th percentile IBW. (C) >75th percentile IBW.
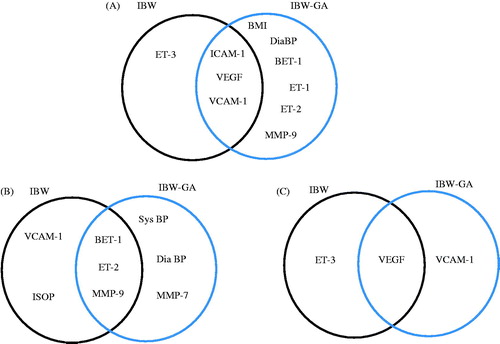
Figure 4. Association of maternal biomarker profiles and (A) infant birth weight. (B) Gestational age.
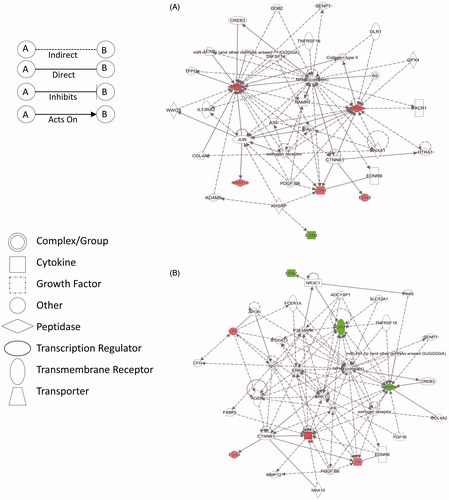
IPA analysis with plasma protein marker levels generated several protein interaction networks. Networks with the highest scores are shown in , for the <25th and >75th percentile groups versus the 25th–75th group. The networks identified are associated with cardiovascular () and inflammatory () disease pathways.
Figure 5. Primary protein networks obtained through ingenuity pathway analysis (IPA) of the fold change of plasma markers in the identified sub groups versus the control group (25th–75th percentile). (A) Network 1 depicting pathways related to cardiovascular disease (<25th percentile) and (B) Network 2 depicting pathways related to inflammatory response (>75th percentile). Red: up-regulation; Green: down-regulation.
Discussion
Our results from this mother-infant cohort study provide interesting and useful preliminary information on maternal biomarkers associated with infant birth weight and gestational age (37–41 weeks). These subsets of MIREC study subjects () appeared to portray the MIREC study population in terms of the distribution of infant birth weight, gestational age and infant birth weight to gestational age ratio values () and also in terms of their maternal systolic and diastolic blood pressure values ().
Maternal circulating endothelins (ET-1 and ET-3), cellular adhesion molecules (ICAM-1 and VCAM-1) and matrix metalloproteinases (MMP-2) exhibited positive associations (p < 0.05) and clustered together in all groups (). Elevated circulating endothelins are linked to endothelial dysfunction (Bátkai et al., Citation2007). It has been reported previously that MMP-2 can cleave precursors of mature peptides ET-1 (e.g. BET-1) and ET-3 (Fernandez-Patron et al., Citation2001). The correlation analysis results reveal that MMP-2 is negatively associated to BET-1 and positively associated to ET-1 (Supplemental ) which supports the notion that the conversion of BET-1 to the potent vasoactive peptide ET-1 can be a result of elevated MMP-2 levels.
It was interesting to note that the lipid oxidation marker 8-isoPGF-2α (ISOP), a marker of oxidative stress, was positively correlated (p < 0.05) with ET-1, ET-2 and ET-3. This is in line with previous reports linking oxidative stress and endothelinergic system (Kahler et al., Citation2001). Also, the lipid oxidation marker was positively correlated (p < 0.05) with maternal BMI, while a negative trend was seen (not significant) with infant birth weight and gestational age.
Correlation analyses also revealed that BET-1, ET-1, ET-2 and ET-3 exhibited a negative relationship (not significant) with infant birth weight (Supplemental ). Meanwhile, maternal plasma ET-1, ET-2 and ET-3 were found to exhibit a positive correlation (not significant) with maternal blood pressure values (Supplemental ). These observations imply that alteration of maternal endothelin homeostasis may influence infant birth weight probably by negatively impacting on maternal blood pressure, and thus possibly intrauterine perfusion. Elevated ET-1 levels and high blood pressure in pregnant women have been shown to cause intrauterine growth restriction that result in low infant birth weights (Arslan et al., Citation2004), consistent with our findings. The ET-2 peptide, similar to ET-1, binds to the ET[A] receptor and mediates vasoconstriction (Schneider et al., Citation2007). The infusion of ET-3 in rats is known to cause a dose-dependent increase in diastolic pressure (Tabrizchi & Triggle, Citation1991).
Our regression analyses revealed that infant birth weight was related to a combination of maternal plasma markers (and ). Of these maternal factors related to birth outcomes, some were common to both IBW and IBW-GA distributions (). Interestingly, both best subsets and polytomous logistic regression analyses yielded similar results for both IBW distributions. In essence, regression analysis of all data collectively suggested that infant birth weight outcome can be associated with the third trimester maternal plasma markers endothelins, ISOP, cellular adhesion molecules, vascular endothelial growth factor (VEGF) and MMP-9. All these maternal plasma markers are implicated in endothelial cell activation, vascular remodeling, oxidative stress and inflammation (Fernandez-Patron et al., Citation2001; Szmitko et al., Citation2003). The clarity in terms of maternal mechanisms was gained when the <25th percentile and >75th percentile groups at both ends of the IBW and IBW-GA distributions were analysed. For instance, across the different approaches in general, low birth weight was consistently associated with elevated maternal plasma BET-1, ET-2 and MMP-9 levels (and ). In contrast, high birth weight was linked to elevated VEGF levels (). These observations indicate that with the <25th percentile group (low birth weight), vasoconstriction pathways may be active, whereas with the >75th percentile group (high birth weight), vasodilation and angiogenesis mechanisms appear to be active. Vascular endothelial growth factor is known to be linked to endothelial NO production and has been associated with enhanced utero-placental perfusion (Valdes et al., Citation2009).
The oxidative stress marker 8-isoPGF-2α (ISOP) was a constituent marker in the model only for the <25th percentile group from the IBW distribution analysis (). This observation is in line with oxidative stress being implicated in low infant birth weight (Fleischer et al., Citation2014).
Moreover, gestational age at birth appeared to be influenced by ET-1, MMP-2 and VEGF based on both regression analyses (and ). In a previous report by Tency et al. (Citation2014). MMPs have been linked to inflammation and are associated with preterm labor. Levels of ET-1 and ET-2 were reported previously to be increased in the amniotic fluid of women with preterm labor and microbial infection of the amniotic cavity (Romero et al., Citation1992). In addition, the ingenuity pathway analysis (IPA) conducted on the maternal plasma markers (expression fold change) for the <25th percentile and >75th percentile groups versus the control groups (25th–75th percentile) yielded two primary networks (). The protein network 1 associated with the low birth weight group (<25th percentile) was related to cardiovascular disease pathway (highest score), especially hypertension. It is interesting to note that in this network ET-1 (EDN1) and MMP-9 are upregulated (). As mentioned before, increased ET-1 is implicated in gestational hypertension (Arslan et al., Citation2004) and increased MMP-9 is seen in women with preterm labor (Tency et al., Citation2014). Meanwhile, the network 2 () associated with high birth weight group (>75th percentile) was related to inflammatory disease pathway (highest score) with increased CRP (red), down-regulated MMP-9 (green) and interaction seen between ET-1 and ET[B] receptor (EDNRB), where ET-1 potentially binds to ET[B] receptor and can upregulate VEGF (red), as seen under hypoxic condition (Spinella et al., Citation2014). Also, our current observations are consistent with our previous findings by high-content proteomic analyses of third trimester plasma from a small subset of mothers from the MIREC cohort where protein interaction networks of inflammation and cardiovascular effects were activated in mothers of low birth weight infants (Kumarathasan et al., Citation2014).
One of the limitations associated with this study is the small sample size. Nevertheless, this work could have benefited from high-content maternal biomarker analyses, and Doppler assessment for information on utero-placental blood flow.
To our knowledge, this is the first work that explores identification of potential maternal biomarkers relevant to lower or higher infant birth weight by integration of multiple maternal plasma markers of key biochemical mechanisms with related maternal physiological parameters (e.g. BP) as an intermediate outcome and pregnancy outcome as a clinical outcome (e.g. IBW), in combination with categorization for regression analyses performed at different levels. Our findings suggest that low infant birth weight is conceivably predominantly influenced by vasoconstrictive and inflammatory mechanisms, while vasodilation pathways can potentially lead to high infant birth weights. Even though some maternal biomarkers (e.g. VCAM-1, ) can appear to be similar to both these infant birth weight groups, the characteristics of related molecular interactions and associated downstream mechanisms can be responsible for orchestrating the different birth outcomes. This is consistent with the report by Wei et al. (Citation2003) where an increased risk for type 2 diabetes, an inflammatory disease in children, was associated with both low and high birth weights, but different molecular phenotypes were shown. Also, pre-eclampsia has been associated with both low and high birth weight babies, suggesting at least two different subsets of patients with pre-eclampsia (Xiong et al., Citation2000).
Candidate biomarkers of oxidative stress, endothelial dysfunction and inflammation identified in this study, especially using established markers of vascular function, the circulating endothelins as anchors, can be useful in future screening of maternal–infant study cohorts. In addition, these maternal markers are also implicated in uteroplacental insufficiency and angiogenesis. This work thus warrants future high-content “proteomic and metabolomic” biomarker analysis in combination with analysis of upstream regulatory events using a systems biology approach for in-depth mechanistic information, especially to identify different molecular phenotypes.
Conclusion
Our findings suggest that maternal molecular mechanisms such as oxidative stress, endothelial dysfunction in the vasculature and inflammation can play a role by affecting vascular remodelling and function in pregnant mothers. This can translate into maternal blood pressure changes (intermediary maternal health outcome) as well as impact on uteroplacental blood flow changes and angiogenesis, and thus can adversely impact on birth outcome. Low and high birth weight outcomes can be due to underlying maternal mechanistic pathways namely, vasoconstriction and vasodilation processes, respectively. These observations imply that future work using systems biology to integrate multiple maternal plasma “OMIC” markers with maternal physiological parameters can advance the understanding of adverse birth outcome pathways.
Supplementary material available online
Supplemental Table
Download MS Word (24.2 KB)Acknowledgements
The authors would like to thank Dr. Anu Saravanamuthu for her technical assistance, as well as Dr. Gurusankar Saravanabhavan and Dr. Ella Atlas for internal review of the manuscript. The authors also acknowledge the valuable contribution of the MIREC participants and the MIREC Study group, especially the site investigators: Peter von Dadelszen, Denise Hemmings, Jingwei Wang, Michael Helewa, Shayne Taback, Mathew Sermer, Warren Foster, Greg Ross, Paul Fredette, Graeme Smith, Mark Walker, Roberta Shear and Linda Dodds.
Declaration of interest
The authors report that they have no conflicts of interest. This work was supported by the Chemicals Management Plan and the Clean Air Regulatory Agenda at Health Canada. The MIREC Research Platform is supported by the Chemicals Management Plan of Health Canada, the Canadian Institutes for Health Research (Grant # MOP – 81285), and the Ontario Ministry of the Environment. This study was supported by Health Canada federal government research funding.
References
- Abu-Saad K, Fraser D. (2010). Maternal nutrition and birth outcomes. Epidemiol Rev 32:5–25
- Akaike H. (1974). A new look at the statistical model identification. IEEE Trans Autom Control 19:716–23
- Alsuwaida A, Mousa D, Al-Harbi A, et al. (2011). Impact of early chronic kidney disease on maternal and fetal outcomes of pregnancy. J Matern Fetal Neonatal Med 24:1432–6
- Arbuckle TE, Fraser WD, Fisher M, et al. (2013). Cohort profile: the maternal-infant research on environmental chemicals research platform. Paediatr Perinat Epidemiol 27:415–25
- Arslan M, Yazici G, Erdem A, et al. (2004). Endothelin 1 and leptin in the pathophysiology of intrauterine growth restriction. Int J Gynaecol Obstet 84:120–6
- Bátkai S, Rajesh M, Mukhopadhyay P, et al. (2007). Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase. Am J Physiol Heart Circ Physiol 293:H909–18
- Bell ML, Ebisu K, Belanger K. (2007). Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect 115:1118–24
- Bielecki A, Saravanabhavan G, Blais E, et al. (2012). An efficient sample preparation method for high-throughput analysis of 15(S)-8-iso-PGF2α in plasma and urine by enzyme immunoassay. J Anal Toxicol 36:595–600
- Burton GJ, Jauniaux E. (2004). Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig 11:342–52
- Centers for Disease Control and Prevention. (2009). Maternal and infant health research: preterm birth. Available at: http://www.cdc.gov/reproductivehealth/maternalinfanthealth/PretermBirth.htm
- Chantrapanichkul P, Chawanpaiboon S. (2013). Adverse pregnancy outcomes in cases involving extremely young maternal age. Int J Gynaecol Obstet 120:160–4
- Cindrova-Davies T. (2009). Gabor Than Award Lecture 2008: pre-eclampsia from placental oxidative stress to maternal endothelial dysfunction. Placenta 30:S55–65
- Conde-Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. (2013). Novel biomarkers for predicting intrauterine growth restriction: a systematic review and meta-analysis. BJOG 120:681–94
- Curhan GC, Willett WC, Rimm EB, et al. (1996). Birth weight and adult hypertension, diabetes mellitus and obesity in US men. Circulation 94:3246–50
- Ferguson KK, Cantonwine DE, Rivera-González LO, et al. (2014). Urinary phthalate metabolite associations with biomarkers of inflammation and oxidative stress across pregnancy in Puerto Rico. Environ Sci Technol 48:7018–25
- Ferguson KK, McElrath TF, Chen YH, et al. (2015a). Repeated measures of urinary oxidative stress biomarkers during pregnancy and preterm birth. Am J Obstet Gynecol 212:208.e1. doi:10.1016/j.ajog.2014.08.007
- Ferguson KK, McElrath TF, Chen YH, et al. (2015b). Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis. Environ Health Perspect 123:210–16
- Fernandez-Patron C, Zouki C, Whittal R, et al. (2001). Matrix metalloproteinases regulate neutrophil–endothelial cell adhesion through generation of endothelin-1[1-32]. FASEB J 15:2230–40
- Fleischer NL, Merialdi M, van Donkelaar A, et al. (2014). Outdoor air pollution, preterm birth, and low birth weight: analysis of the world health organization global survey on maternal and perinatal health. Environ Health Perspect 122:425–30
- Gelson E, Johnson M. (2010). Effect of maternal heart disease on pregnancy outcomes. Expert Rev Obstet Gynecol 5:605–17
- Gitto E, Reiter RJ, Karbownik M, et al. (2002). Causes of oxidative stress in the pre-and perinatal period. Biol Neonate 81:146–57
- Guo Y, Weck J, Sundaram R, et al. (2014). Urinary concentrations of phthalates in couples planning pregnancy and its association with 8-hydroxy-2'-deoxyguanosine, a biomarker of oxidative stress: longitudinal investigation of fertility and the environment study. Environ Sci Technol 48:9804–11
- Hackshaw A, Rodeck C, Boniface S. (2011). Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Hum Reprod Update 17:589–604
- Howe LD, Matijasevich A, Tilling K, et al. (2012). Maternal smoking during pregnancy and offspring trajectories of height and adiposity: comparing maternal and paternal associations. Int J Epidemiol 41:722–32
- JAMA. (2002). JAMA patient page: low birth weight. J Am Med Assoc 287:270
- Jolly M, Sebire N, Harris J, et al. (2000). The risks associated with pregnancy in women aged 35 years or older. Hum Reprod 15:2433–7
- Kahler J, Ewert A, Weckmuller J, et al. (2001). Oxidative stress increases endothelin-1 synthesis in human coronary artery smooth muscle cells. J Cardiovasc Pharmacol 38:49–57
- Kenny LC, Lavender T, McNamee R, et al. (2013). Advanced maternal age and adverse pregnancy outcome: evidence from a large contemporary cohort. PLoS One 8:e56583
- Kramer MS, Platt RW, Wen SW, et al. (2001). A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics 108:E35
- Kumarathasan P, Goegan P, Vincent R. (2001). An automated high-performance liquid chromatography fluorescence method for the analyses of endothelins in plasma samples. Anal Biochem 299:37–44
- Kumarathasan P, Vincent R, Das D, et al. (2014). Applicability of a high-throughput shotgun plasma protein screening approach in understanding maternal biological pathways relevant to infant birth weight outcome. J Proteomics 100:136–46
- Lawlor DA, Macdonal-Wallis C, Fraser A, et al. (2012). Cardiovascular biomarkers and vascular function during childhood in the offspring of mothers with hypertensive disorders of pregnancy: findings from the Avon Longitudinal Study of Parents and Children. Eur Heart J 33:335–45
- Mathews F, Yudkin P, Neil A. (1999). Influence of maternal nutrition on outcome of pregnancy: prospective cohort study. BMJ 319:339–43
- Murray CJL, Lopez AD. (1997). Mortality by cause for eight regions of the world: global burden of disease study. Lancet 349:1269–76
- Neter J, Wasserman W, Kutner M. (1985). Applied linear regression models. 2nd ed. Homewood, IL: Irwin
- Olsson D, Mogren I, Forsberg B. (2013). Occupational and environmental medicine air pollution exposure in early pregnancy and adverse pregnancy outcomes: a register-based cohort study. BMJ Open 3:e001955
- Perera FP, Tang D, Rauh V, et al. (2007). Relationship between polycyclic aromatic hydrocarbon-DNA adducts, environmental tobacco smoke, and child development in the World Trade Center cohort. Environ Health Perspect 115:1497–502
- Perinatal health indicators for Canada 2013, a report from the Canadian perinatal surveillance system. Public Health Agency of Canada, 2013. Available at: http://publications.gc.ca/collections/collection_2014/aspc-phac/HP7-1-2013-eng.pdf [last accessed 17 Sep 2014]
- Romero R, Avila C, Edwin SS, Mitchell MD. (1992). Endothelin-1,2 levels are increased in the amniotic fluid of women with preterm labor and microbial invasion of the amniotic cavity. Am J Obstet Gynecol 166:95–9
- Ross JA. (2006). High birthweight and cancer: evidence and implications. Cancer Epidemiol Biomarkers Prev 15:1–2
- Schneider MP, Boesen EI, Pollock DM. (2007). Contrasting actions of endothelin ET(A) and ET(B) receptors in cardiovascular disease. Annu Rev Pharmacol Toxicol 47:731–59
- Sharnkardass K, O’campo P, Dodds L, et al. (2014). Magnitude of income-related disparities in adverse perinatal outcomes. BMC Pregnancy Childbirth 14:96
- Spinella F, Caprara V, Cianfrocco R, et al. (2014). The interplay between hypoxia, endothelial and melanoma cells regulates vascularization and cell motility through endothelin-1 and vascular endothelial growth factor. Carcinogenesis 5:840–8
- Stangl V, Schad J, Gossing G, et al. (2008). Maternal heart disease and pregnancy outcome: a single-centre experience. Eur J Heart Fail 10:855–60
- Stillerman KP, Mattison DR, Giudice LC, Woodruff TJ. (2008). Environmental exposures and adverse pregnancy outcomes: a review of the science. Reprod Sci 15:631–50
- Szmitko PE, Wang CH, Weisel RD, et al. (2003). New markers of inflammation and endothelial cell activation: Part I. Circulation 108:1917–23.21
- Tabacova S. (2000). Adverse pregnancy outcomes associated with oxidized nitrogen exposures and oxidative stress: human and animal evidence. Annual Conference of the ISEE. Epidemiology 11:S143
- Tabacova S, Baird DD, Balabaeva L. (1998). Exposure to oxidized nitrogen: lipid peroxidation and neonatal health risk. Arch Environ Health 53:214–21
- Tabrizchi R, Triggle CR. (1991). Effects of endothelin 3 on diastolic blood pressure of pithed Sprague–Dawley, Wistar Kyoto, and spontaneously hypertensive rats before and after pretreatment with nifedipine. Can J Physiol Pharmacol 69:531–5
- Tency I, Temmerman M, Vaneechoutte M. (2014). Inflammatory response in maternal serum during preterm labour. Facts Views Vis Obgyn 6:19–30
- Tetz LM, Cheng AA, Korte CS, et al. (2013). Mono-2-ethylhexyl phthalate induces oxidative stress responses in human placental cells in vitro. Toxicol Appl Pharmacol 268:47–54
- Triche EW, Hossain N. (2007). Environmental factors implicated in the causation of adverse pregnancy outcome. Semin Perinatol 31:240–2
- Valdes G, Kaufmann P, Corthorn J, et al. (2009). Vasodilator factors in the systemic and local adaptations to pregnancy. Reprod Biol Endocrinol 7:79
- Watkins DJ, Ferguson KK, Anzalota Del Toro LV, et al. (2015). Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in Puerto Rico. Int J Hyg Environ Health 218:212–19
- Wei JN, Lin RS, Sung FC, et al. (2003). Low birth weight and high birth weight infants are both at an increased risk to have type 2 diabetes among schoolchildren in Taiwan. Diabetes Care 26:343–8
- Wigle DT, Arbuckle TE, Turner MC, et al. (2008). Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J Toxicol Environ Health B Crit Rev 11:373–517
- Woodruff TJ, Parker JD, Darrow LA, et al. (2009). Methodological issues in studies of air pollution and reproductive health. Environ Res 109:311–20
- World Health Organization. World Health Statistics 2012. Available at: http://apps.who.int/iris/bitstream/10665/44844/1/9789241564441_eng.pdf
- Xiong X, Demianczuk NN, Buekens P, Saunders LD. (2000). Association of preeclampsia with high birth weight for age. Am J Obstet Gynecol 183:148–55
