Abstract
Context Matrix metalloproteinases (MMPs) are associated with diastolic dysfunction and heart failure in acquired heart disease.
Objective To investigate the role of MMPs as novel biomarkers in clinically stable adults with congenital heart disease.
Methods We measured serum MMP-2, -3, -9 and tissue inhibitor of matrix metalloproteinase-1 in 425 patients and analysed the association with cardiac function and exercise capacity.
Results MMP-2 was significantly associated with exercise capacity, ventilatory efficiency and left ventricular deceleration time, independently of age, sex, body surface area and NT-proBNP.
Conclusion MMP-2 may provide new information in the clinical evaluation of adults with congenital heart disease.
Introduction
Since Jerome Gross and Charles Lapiere initially described the enzyme collagenase while observing the metamorphosis of the tadpole in 1962, much research has been performed on what is nowadays known as matrix metalloproteinase-1 (Gross & Lapiere, Citation1962). Many other members of the family of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs), consisting of structurally and functionally related extracellular proteolytic enzymes, have been described (Visse & Nagase, Citation2003). Together, these enzymes are capable of degrading all kinds of extracellular matrix protein components (Bode et al., Citation1999; Stetler-Stevenson et al., Citation1993). A disruption of the balance between MMPs and TIMPs is observed in a wide variety of pathological conditions, such as acute and chronic cardiovascular diseases, cancer progression and different types of arthritis (Baker et al., Citation2002; Bode et al., Citation1999; Jones et al., Citation2008; Konttinen et al., Citation1999). More specific, altered MMP-2, MMP-9 and TIMP-1 levels have been associated with left ventricular (LV) hypertrophy, chronic heart failure and diastolic dysfunction in hypertensive heart disease and coronary artery disease (Ahmed et al., Citation2006; Chu et al., Citation2013; Kang et al., Citation2008; Laviades et al., Citation1998; Martos et al., Citation2007). In patients with thoracic aortic aneurysms, specific MMP-2, -9 and TIMP-1 profiles have been described; however, the majority of research in this field is based on histology of the aortic wall rather than circulating blood biomarker levels (Ikonomidis et al., Citation2013; Wang et al., Citation2016; Zhang et al., Citation2014).
To date, little is known about circulating MMP profiles in adults with congenital heart disease (CHD). As biventricular fibrosis, hypertrophy and dysfunction are important causes of morbidity and mortality, the evaluation of MMPs may well prove to be important in this setting. Therefore, the goal of this study was to evaluate MMP profiles in adult CHD and to investigate its relation with exercise capacity and echocardiographic findings, in order to explore the potential role of MMPs as new biomarkers.
Methods
Study design
This is a cross-sectional, observational study. Patients who routinely visited our adult congenital cardiology outpatient clinic between May 2011 and April 2013 were approached and prospectively enrolled. At the same day, patients underwent physical examination by a cardiologist, 12-lead electrocardiography, echocardiography and venous blood sampling. A minority of patients underwent exercise testing for routine clinical follow-up during the same week as the other study investigations. According to the study protocol, these exercise testing results were collected if performed as routine clinical care. The study protocol was approved by the institutional review board of the Erasmus MC and written informed consent was obtained from all subjects.
The following congenital cardiac diagnoses were included: repaired tetralogy of Fallot (including pulmonary atresia with ventricular septal defect), congenital aortic stenosis, aortic coarctation, transposition of the great arteries (TGA) corrected by atrial switch operation (Mustard/Senning), TGA corrected by arterial switch operation, congenitally corrected TGA and functionally univentricular hearts palliated by Fontan procedure. Exclusion criteria were: age <18 years, severe renal impairment (creatinine >200 μmol/L), pregnancy and mild cardiac lesions (isolated atrial or ventricular septal defect).
Data collection
Patients were anonymised using a central online coding system. All data collection was performed using an electronic CRF-based online system (© 2004-2012 OpenClinica, LLC and collaborators, Waltham, MA; www.OpenClinica.com). This included patient demographics, medical history, medication use, symptoms and signs of heart failure (New York Heart Association (NYHA) classification), results of physical examination, electrocardiography, echocardiography, exercise capacity and laboratory results.
Echocardiography
Patients underwent two-dimensional transthoracic echocardiography using a commercially available ultrasound system iE33 (Philips Medical Systems, Best, The Netherlands) with a 1.5-MHz transducer. Cardiac dimensions and function were measured in accordance with the most recent recommendations of the American Society of Echocardiography and the European Association of Cardiovascular Imaging (Lang et al., Citation2015). Left ventricular (LV) and right ventricular (RV) dimensions were measured in end-diastolic phase from the parasternal long-axis and RV-focused apical four-chamber view, respectively. LV ejection fraction was calculated using the biplane method of disc summation (modified Simpson’s rule). RV systolic function was quantified by tricuspid annular plane systolic excursion (TAPSE) and RV fractional area change. Additionally, LV and RV systolic function was qualitatively graded as normal or abnormal. LV diastolic function was measured using pulsed wave Doppler echocardiography of the mitral valve inflow (E, A and deceleration time) and tissue Doppler imaging of the medial mitral annulus velocity from the apical four-chamber view (E'). Echocardiographic measurements of patients with a systemic (sub-aortic) RV were assessed similar to patients with a sub-pulmonary RV.
Exercise capacity
Exercise testing was performed on an electrically braked cycle ergometer (ergoselect 200 P, ergoline GmbH, Bitz, Germany), using a gradually increasing workload of 20 W/min. It was aimed to complete the test within 8–12 min. Minute ventilation, oxygen uptake and carbon dioxide elimination were measured using a computerised breath-by-breath analyser. Maximum effort was defined as a peak respiratory exchange ratio of >1.10. Peak workload, heart rate and oxygen uptake were expressed as percentage of the predicted value, based on age, gender, body height and weight. Ventilatory efficiency was expressed as carbon dioxide equivalent (minute ventilation relative to carbon dioxide elimination).
Biomarkers
Venous blood samples were obtained after 30 min of rest. Plasma N-terminal-pro-B-type natriuretic peptide (NT-proBNP) was assessed by electrochemiluminescence immunoassays (Roche Diagnostics, Basel, Switzerland) in our clinical chemistry laboratory, as part of routine patient care. Other blood samples were coded, processed and stored at –80 °C within 2 h, until further analysis. Serum MMP-2, MMP-3 and MMP-9 were measured by batch analysis using the ProcartaPlexTM Multiplex Immunoassay, with sample dilution factor 50 (eBioscience, Vienna, Austria). Serum TIMP-1 was measured using two-site enzyme-linked immunosorbent assays, with sample dilution factor 100 (BMS2018CE, eBioscience, Vienna, Austria). Lower limits of detection were <34 pg/mL, <0.83 pg/mL and <39 ng/mL for MMP-2, MMP-9 and TIMP-1, respectively. As all blood samples were coded, laboratory staff was blinded for patient data.
Statistical analysis
The distribution of data was checked using histograms and the Shapiro–Wilk test. Patient characteristics were presented as mean ± standard deviation or median [interquartile 1–3 (IQ1–IQ3)], depending on the distribution of data. Skewed biomarker distributions were log transformed.
Univariable associations were evaluated using Pearson’s correlation. MMP values below the limit of detection were analysed as the lowest detectable value. We performed a stratified analysis in the three main diagnostic groups (left-sided heart disease, right-sided heart disease and systemic RV).
Multivariable linear (for continuous outcomes) and logistic (for dichotomous outcomes) regression analyses were only performed for MMP-2, as all other biomarker values yielded non-significant results in the univariable analysis. Unstandardised β-coefficients were adjusted for the potential confounders age, sex and body surface area (BSA). Based on the results of the univariable analysis, β-coefficients were additionally adjusted for other covariates. Data analysis was performed using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY). p Values <0.05 were considered statistically significant.
Results
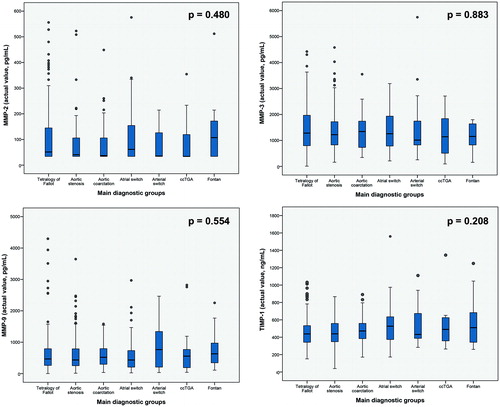
We prospectively included 425 patients (median age 33.3 years [IQ1–IQ3 25.9–42.1 years], 244 males, 57%). Echocardiography and measurement of serum MMPs was performed in all patients. Bicycle ergometry was performed in 126 patients, of whom 40 patients underwent measurement of oxygen uptake and carbon dioxide elimination. Clinical characteristics and medication use are detailed in . MMP and TIMP profiles were not different when comparing all patient subgroups based on the congenital cardiac diagnosis ().
Table 1. Characteristics of study population (n = 425).
Univariable analysis
In , the correlations of 10log MMP-2, MMP-3, MMP-9 and TIMP-1 with patient characteristics, exercise testing results and echocardiographic data are presented. MMP-2 and MMP-9 were significantly related to BSA, diastolic blood pressure and decreased saturation. MMP-2 significantly correlated with peak workload % (r = −0.233, p = 0.009), peak oxygen uptake (r = −0.411, p = 0.008) and carbon dioxide equivalent (r = 0.396, p = 0.034), of which scatterplots are additionally shown in . Furthermore, MMP-2 significantly correlated to NT-proBNP, NYHA functional class, aortic valve peak velocity, LV and RV systolic dysfunction, E' wave velocity <8 cm/s, E/E' ratio ≥13 and LV deceleration time. MMP-9 was only related to peak oxygen uptake (r = −0.350, p = 0.027). MMP-3, MMP-9, TIMP-1 and MMP-9/TIMP-1 ratios (ratios are not presented in ) were not significantly associated with any other parameters of functional outcome. As 46% of MMP-2 values were <34 pg/mL (lower limit of detection), all analyses were repeated with exclusion of these values. Non-parametric tests were also performed (Spearman’s correlation). These did not yield different conclusions.
Figure 2. Scatterplots demonstrating the correlation of MMP-2 with peak workload %, peak oxygen uptake and carbon dioxide equivalent.
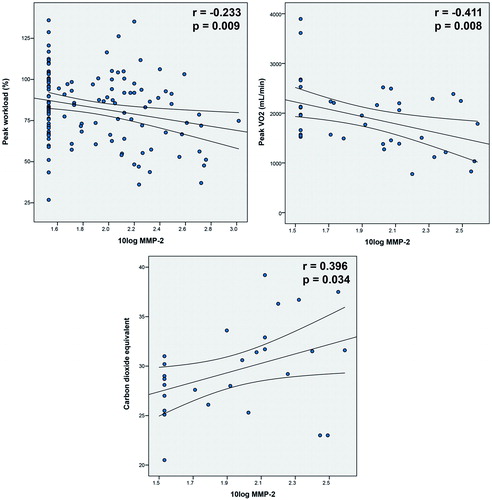
Table 2. Univariable associations (Pearson's correlation, n = 425).
Stratified analysis
The associations of echocardiographic measurements with MMP-2 as presented in were additionally analysed in three main diagnostic subgroups: left-sided heart disease (congenital aortic stenosis, aortic coarctation and TGA corrected by arterial switch operation), right-sided heart disease (repaired tetralogy of Fallot), and systemic RV (TGA corrected by atrial switch operation and congenitally corrected TGA). In patients with repaired tetralogy of Fallot, LV dysfunction (r = 0.184, p = 0.018), RV dysfunction (r = 0.190, p = 0.014), E/E' ratio ≥13 (r = 0.165, p = 0.042) and LV deceleration time (r = −0.178, p = 0.028) were significantly associated with MMP-2. These associations were not present in the two other diagnostic groups, and LV diastolic function was not measured in patients with a systemic RV.
Multivariable analysis
In , the results of the multivariable analysis are presented. Β-coefficients represent the increase in patient characteristic per 10-fold increase in MMP-2, adjusted for age, sex and BSA. Based on the results of the univariable analysis, we additionally adjusted for diastolic blood pressure, decreased saturation and NT-proBNP (not shown in the table). After adjustment for all variables, MMP-2 was significantly associated with peak workload (β = −10.96, p = 0.022), carbon dioxide equivalent (β = −6.86, p = 0.016) and LV deceleration time (β = −22.4 ms, p = 0.012); however, not with RV dilatation and RV/LV systolic function. MMP-2 was not significantly associated with aortic sinotubular junction end-diastolic diameter, although a trend was observed (β = 1.89, p = 0.087).
Table 3. Unstandardised B-coefficients of 10log MMP-2 values; analysed using linear (for continuous outcome) or logistic regression (for categorical outcome), n = 425.
Discussion
In our cohort of clinically stable adults with congenital heart disease, MMP-2 was significantly associated with decreased exercise capacity (n = 126, peak workload %), decreased ventilatory efficiency (n = 40, carbon dioxide equivalent) and decreased LV deceleration time (n = 425), independently of NT-proBNP and other clinical variables. In addition, MMP-2 was related to LV and RV systolic dysfunction (adjusted for age, sex and BSA) and several echocardiographic markers of diastolic dysfunction in univariable analysis. MMP-3, MMP-9 and TIMP-1 were not consistently related with exercise capacity or cardiac function. Nor did we observe specific MMP profiles among the varying underlying congenital diagnoses.
Cardiac function
Although this is the first study that reports a modest association of MMP-2 with systolic ventricular function, independently of age, sex and BSA, extracellular matrix remodelling processes have been previously related to systolic ventricular function in other patient groups. In patients with hypertension, serum TIMP-1 levels were independently related to LV longitudinal strain and torsion (Kang et al., Citation2008). Moreover, TIMP-1 was elevated in patients with congestive heart failure (Ahmed et al., Citation2006).
Increased extracellular matrix remodelling within the myocardium has been more frequently associated with diastolic ventricular dysfunction. In our cohort, diastolic dysfunction (as expressed by E' wave velocity <8 cm/s, E/E' ratio ≥13 and LV deceleration time) was related to MMP-2 in univariable analysis. Martos et al. (Citation2007) showed that in hypertensive heart disease, serum levels of MMP-2 were elevated in those patients with diastolic heart failure, suggesting increased degradation of myocardial collagen and other components of the extracellular matrix. Subsequently, Gardin et al. (Citation2009) showed that echocardiographic markers of diastolic dysfunction are independently related to peak oxygen uptake and ventilatory efficiency. In line with these results, Chen et al. (Citation2013) recently reported that a serum biomarker of collagen type I synthesis (carboxy-terminal propeptide of procollagen type I, PICP) was related to higher RV late gadolinium enhancement scores (indicating myocardial fibrosis) and lower peak oxygen uptake in patients with repaired tetralogy of Fallot. Diastolic function is often impaired in adults with CHD (Eindhoven et al., Citation2013). Diastolic dysfunction is especially common in patients with congenital aortic stenosis (Dusenbery et al., Citation2014; Friedman et al., Citation2013). In addition, abnormal LV diastolic indices are known to be prevalent in a substantial proportion of patients with tetralogy of Fallot (Aboulhosn et al., Citation2013). The most important association found in our study, directly linking increased serum MMP-2 values with decreased exercise capacity and ventilatory efficiency, may therefore be partially explained by increased diastolic dysfunction.
Importantly, the patients in our study were all clinically stable, with 91% of patients in NYHA class I. This probably explains some of the only modest or absent correlations. The stratified analysis in three main diagnostic groups showed that the associations of echocardiographic measurements with MMP-2 as found in the entire study cohort probably largely originate from the group of patients with repaired tetralogy of Fallot. This is possibly explained by a wider variance in ventricular systolic and diastolic function in this patient group.
Aortic stenosis and dilatation
In our study, serum MMP-2, -3, -9 and TIMP-1 levels were similar in patients with aortic stenosis or coarctation compared with all other congenital cardiac diagnoses. As we know patients with aortic stenosis or coarctation often have diastolic dysfunction, we had expected higher MMP-2 levels in these patients (Eindhoven et al., Citation2013). Neither did we find significant relations with aortic dimensions as measured by echocardiography, although a trend was observed for MMP-2 (adjusted p value 0.087). Previous studies have reported altered plasma MMP-2, -9 and TIMP-1 levels in patients with aortic valve stenosis and ascending aorta dilatation, both in bicuspid valves and in chronic thoracic aortic dissection (Ikonomidis et al., Citation2013; Wang et al., Citation2016; Zhang et al., Citation2014). It may therefore be worthwhile to evaluate MMPs in specific patient subgroups with a high risk of aortic dilatation, in combination with more detailed imaging modalities of the aorta, such as CT or MRI.
MMPs as peripheral markers
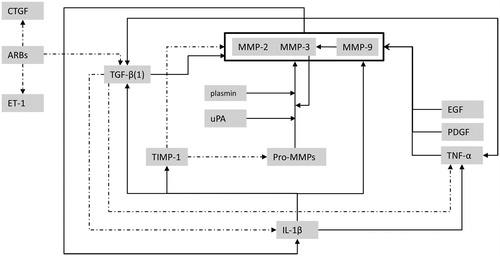
As circulating blood biomarkers profiles can be affected by processes throughout the entire body, altered MMP profiles may not entirely reflect the actual histological changes in the myocardium or aortic wall. The interaction between MMPs and other biologically active substrates is highly complex. We constructed a conceptual model of the MMP–TIMP interactions with TGF-β, IL-1β, TNF-α and other substrates as described by the available literature on this topic () (Brown et al., Citation2007; Friedberg et al., Citation2013; Jones et al., Citation2008; Stetler-Stevenson et al., Citation1993; Vaday et al., Citation2001). Due to these complex interactions, it is likely that the underlying disease and patient comorbidities influence MMP levels. In this study, MMP-9 and TIMP-1 were not found to be associated with echocardiographic data, in contrast to previous reports (Ahmed et al., Citation2006; Chu et al., Citation2013; Martos et al., Citation2007). This might be explained by the difference in patient cohorts, the selection of stable patients visiting the outpatient clinic and the type of MMP analysis performed.
Figure 3. Conceptual model illustrating the highly complex interactions between MMPs, TIMP-1 and other biologically active substrates (dashed lines indicate enzyme inhibition). ARBs, angiotensin receptor blockers; CTGF, connective tissue growth factor; EGF, epidermal growth factor; ET-1, endothelin-1; IL-1β, interleukin-1beta; PDGF, platelet derived growth factor; TGF-β, transforming growth factor-beta; TNF-α, tumour necrosis factor-alpha; uPA, urokinase-type plasminogen activator.
Clinical implications
Previous studies have shown that exercise capacity and ventilatory response to exercise are important prognostic markers in adult CHD (Diller et al., Citation2005; Dimopoulos et al., Citation2006). Our data provide evidence for a modest association of MMP-2 with several measures of functional outcome, independently of NT-proBNP. Correlations were not strong, indicating that MMP-2 only partially explains the variance in echocardiographic findings and exercise capacity. Nevertheless, these findings indicate that MMP-2 provides new information, incremental to clinical variables and NT-proBNP, even in a clinically stable patient group. MMP-2 may therefore be useful as novel biomarker in adults with CHD. It would be worthwhile to further evaluate the prognostic value of MMP-2 in longitudinal studies.
Study limitations
Bicycle ergometry was performed in a subset of 126 patients. Of these, in 40 patients oxygen uptake and carbon dioxide elimination were recorded. The consistency and strength of these data should therefore be further evaluated in multiple studies from different centres and laboratories, in accordance with previously formulated benchmarks for the assessment of novel cardiovascular biomarkers by Morrow and de Lemos (Citation2007). To be clinically useful, the preanalytical and analytical performance of the MMP assays need to be thoroughly investigated and robust reference values have to be assessed in healthy controls.
Conclusion
Serum MMP-2 was significantly related to exercise capacity, ventilatory efficiency and LV deceleration time in a diverse cohort of stable adults with congenital heart disease, also after adjustment for NT-proBNP and other clinical variables. In contrast, MMP-3, MMP-9 and TIMP-1 were not consistently related to exercise testing results or cardiac function. Prospective longitudinal studies are warranted to determine the clinical usefulness and prognostic value of MMP-2 as candidate biomarker.
Acknowledgements
We thank all the laboratory staff of the Erasmus MC who contributed to this project. The many hours of dedicated work they spent on the processing and analysis of blood samples were essential for the execution of this study.
Disclosure statement
The authors report no declarations of interest.
References
- Aboulhosn JA, Lluri G, Gurvitz MZ, et al. (2013). Left and right ventricular diastolic function in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Can J Cardiol 29:866–72.
- Ahmed SH, Clark LL, Pennington WR, et al. (2006). Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation 113:2089–96.
- Baker AH, Edwards DR, Murphy G. (2002). Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci 115:3719–27.
- Bode W, Fernandez-Catalan C, Grams F, et al. (1999). Insights into MMP-TIMP interactions. Ann N Y Acad Sci 878:73–91.
- Brown RD, Jones GM, Laird RE, et al. (2007). Cytokines regulate matrix metalloproteinases and migration in cardiac fibroblasts. Biochem Biophys Res Commun 362:200–5.
- Chen CA, Tseng WY, Wang JK, et al. (2013). Circulating biomarkers of collagen type I metabolism mark the right ventricular fibrosis and adverse markers of clinical outcome in adults with repaired tetralogy of Fallot. Int J Cardiol 167:2963–8.
- Chu JW, Jones GT, Tarr GP, et al. (2013). Plasma active matrix metalloproteinase 9 and indices of diastolic function in patients with preserved systolic function. Int J Cardiol 167:1242–6.
- Diller GP, Dimopoulos K, Okonko D, et al. (2005). Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation 112:828–35.
- Dimopoulos K, Okonko DO, Diller GP, et al. (2006). Abnormal ventilatory response to exercise in adults with congenital heart disease relates to cyanosis and predicts survival. Circulation 113:2796–802.
- Dusenbery SM, Jerosch-Herold M, Rickers C, et al. (2014). Myocardial extracellular remodeling is associated with ventricular diastolic dysfunction in children and young adults with congenital aortic stenosis. J Am Coll Cardiol 63:1778–85.
- Eindhoven JA, van den Bosch AE, Ruys TP, et al. (2013). N-terminal pro-B-type natriuretic peptide and its relationship with cardiac function in adults with congenital heart disease. J Am Coll Cardiol 62:1203–12.
- Friedberg MK, Cho MY, Li J, et al. (2013). Adverse biventricular remodeling in isolated right ventricular hypertension is mediated by increased transforming growth factor-beta1 signaling and is abrogated by angiotensin receptor blockade. Am J Respir Cell Mol Biol 49:1019–28.
- Friedman KG, Mcelhinney DB, Rhodes J, et al. (2013). Left ventricular diastolic function in children and young adults with congenital aortic valve disease. Am J Cardiol 111:243–9.
- Gardin JM, Leifer ES, Fleg JL, et al. (2009). Relationship of Doppler-Echocardiographic left ventricular diastolic function to exercise performance in systolic heart failure: the HF-ACTION study. Am Heart J 158:S45–52.
- Gross J, Lapiere CM. (1962). Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci USA 48:1014–22.
- Ikonomidis JS, Ivey CR, Wheeler JB, et al. (2013). Plasma biomarkers for distinguishing etiologic subtypes of thoracic aortic aneurysm disease. J Thorac Cardiovasc Surg 145:1326–33.
- Jones JA, Mcnally AK, Chang DT, et al. (2008). Matrix metalloproteinases and their inhibitors in the foreign body reaction on biomaterials. J Biomed Mater Res A 84:158–66.
- Kang SJ, Lim HS, Choi BJ, et al. (2008). Longitudinal strain and torsion assessed by two-dimensional speckle tracking correlate with the serum level of tissue inhibitor of matrix metalloproteinase-1, a marker of myocardial fibrosis, in patients with hypertension. J Am Soc Echocardiogr 21:907–11.
- Konttinen YT, Ainola M, Valleala H, et al. (1999). Analysis of 16 different matrix metalloproteinases (MMP-1 to MMP-20) in the synovial membrane: different profiles in trauma and rheumatoid arthritis. Ann Rheum Dis 58:691–7.
- Lang RM, Badano LP, Mor-Avi V, et al. (2015). Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 28:1–39 e14.
- Laviades C, Varo N, Fernandez J, et al. (1998). Abnormalities of the extracellular degradation of collagen type I in essential hypertension. Circulation 98:535–40.
- Martos R, Baugh J, Ledwidge M, et al. (2007). Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation 115:888–95.
- Morrow DA, de Lemos JA. (2007). Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation 115:949–52.
- Stetler-Stevenson WG, Liotta LA, Kleiner DE. JR. (1993). Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J 7:1434–41.
- Vaday GG, Schor H, Rahat MA, et al. (2001). Transforming growth factor-beta suppresses tumor necrosis factor alpha-induced matrix metalloproteinase-9 expression in monocytes. J Leukoc Biol 69:613–21.
- Visse R, Nagase H. (2003). Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92:827–39.
- Wang Y, Wu B, Dong L, et al. (2016). Circulating matrix metalloproteinase patterns in association with aortic dilatation in bicuspid aortic valve patients with isolated severe aortic stenosis. Heart Vessels 31:189–97.
- Zhang X, Wu D, Choi JC, et al. (2014). Matrix metalloproteinase levels in chronic thoracic aortic dissection. J Surg Res 189:348–58.