Abstract
Objectives To evaluate the impact of a 91-day extended regimen combined oral contraceptive (150 μg levonorgestrel [LNG]/30 μg ethinylestradiol [EE] for 84 days, followed by 10 μg EE for seven days [Treatment 1]) compared with two traditional 21/7 regimens (21 days 150 μg LNG/30 μg EE [Treatment 2] or 150 μg desogestrel [DSG]/30 μg EE [Treatment 3], both with seven days’ hormone free), on several coagulation factors and thrombin formation markers.
Methods Randomised, open-label, parallel-group comparative study involving healthy women (18–40 years). The primary endpoint was change from baseline in prothrombin fragment 1 + 2 (F1 + 2) levels over six months.
Results A total of 187 subjects were included in the primary analysis. In all groups, mean F1 + 2 values were elevated after six months of treatment. Changes were comparable between Treatments 1 and 2 (least squares mean change: 170 pmol/L and 158 pmol/L, respectively) but noticeably larger after Treatment 3 (least squares mean change: 592 pmol/L). The haemostatic effects of Treatment 1 were comparable to those of Treatment 2 and noninferior to those of Treatment 3 (lower limit of 95% confidence interval [− 18.3 pmol/L] > − 130 pmol/L).
Conclusions The LNG/EE regimens had similar effects on F1 + 2. Noninferiority was demonstrated between extended regimen LNG/EE and DSG/EE.
Chinese Abstract
摘 要 目的 评估口服避孕药91天长周期疗法(150μg左炔诺孕酮[levonorgestrel,LNG]/30μg炔雌醇[ethinylestradiol,EE]84天,10μg EE 7天[治疗1])与两种21/7常规方案(21天150μg LNG/30μg EE [治疗2]或150μg去氧孕烯[desogestrel,DSG]/30μg EE [治疗3],两种治疗均有7天的间歇期)对凝血因子和凝血酶形成标记物的影响。
方法 关于18-40岁健康女性的随机、开放、平行组对照研究。主要研究终点为治疗6个月后凝血酶原片段F1+2与基线水平的变化。
结果 初步分析了187名受试者,治疗6个月后整个人群的F1+2平均水平上升。治疗1与治疗2的变化类似:最小二乘均数变化分别为170 pmol/L和158 pmol/L,但治疗3的变化明显大:最小二乘均数变化为592 pmol/L。治疗1的止血效果与治疗2的相似,但不比治疗3差(95%置信区间下限[ -18.3 pmol/L] > -130 pmol/L)。
结论 LNG/EE两种治疗方案对F1+2的影响相似,LNG/EE长周期方案与DSG/EE常规方案相比无差别。
INTRODUCTION
Treatment with combined oral contraceptives (COCs) is associated with an increased risk of venous thromboembolism (VTE) caused by changes in the procoagulant, anticoagulant, and fibrinolytic pathways of blood coagulationCitation1. The increase in both procoagulatory and fibrinolytic activity observed during treatment with most COCs indicates a shift of the equilibrium between coagulation and fibrinolysis to a higher level of fibrin turnover. These changes have been attributed to the effects of the oestrogen component, particularly on fibrinogen, factor VII, antithrombin, protein S, plasminogen and tissue plasmogen activator (t-PA). The higher level of fibrin turnover leads to an enhanced formation of prothrombin fragments 1 + 2 (F1 + 2) and D-dimers, as well as thrombin-antithrombin complex and plasmin-antiplasmin complexCitation2.
Historically, the link between COCs and VTE has depended on the dose of the oestrogen component (typically supplied as ethinylestradiol [EE]). However, evidence suggests that the incidence of VTE with low-dose COCs varies with the type of progestin and it appears to be up to two-fold lower for pills containing second-generation progestins like levonorgestrel (LNG) than for those releasing third-generation progestins such as desogestrel (DSG) and gestodeneCitation1,Citation3–6. COCs containing different progestin induce different sensitivities to activated protein C, which may account for the variation in thrombotic risk among COC formulationsCitation7,Citation8. Women on the lowest combined dose of LNG and EE tend to be less affected by adverse changes in coagulation variables than users of other COC preparationsCitation9.
The risk of VTE has been reported to be highest in the first months of COC use, as the most dramatic changes in haemostatic indices are observed during the first cycle of exposureCitation10. Prolonged duration of COC use does not impact VTE risk, which is immediate, reversible, noncumulative, and rapidly decreases within three months after stoppingCitation11,Citation12.
The 91-day extended regimen consists of 150 μg LNG and 30 μg EE for 84 days, followed by seven days of 10 μg of EE in place of placebo or no treatment. This COC is a modification of the traditional 21/7 regimen in which the number of days of combined active tablets administered is increased, resulting in fewer scheduled withdrawal bleeding episodes per year. Certain data suggest that two-thirds of women would choose to have a menstrual period every three months or lessCitation13; therefore, a regimen associated with fewer withdrawal bleeding episodes may also be favoured by many women. Fewer hormone-free intervals (HFIs) should lead to a reduction in the occurrence of common hormone withdrawal symptoms associated with 21/7 regimens, such as breast tenderness and bloatingCitation14. Extended regimen COCs may also be used to treat conditions such as endometriosis and dysmenorrhoeaCitation14. Further, addition of low-dose oestrogen to an extended regimen COC during the HFI improves bleeding profiles while still providing effective prevention of pregnancyCitation15.
Although the FDA-approved 91-day COC Seasonique® (Teva Branded Pharmaceutical Products R&D, Inc., Frazer, PA) has been available in the United States since 2006 and in Canada since 2010, no formal study has investigated its effect on haemostatic parameters. To address this need, we initiated a randomised, open-label, parallel-group study to compare the impact of Seasonique® on several coagulation factors and markers of thrombin formation with those of two traditional 21/7-day regimen COCs.
METHODS
This was a multinational, multicentre, open-label, randomised trial designed to evaluate the haemostatic effects of three COC regimens in women of child-bearing age. The study was conducted between 23 November 2010 and 2 December 2011, at 22 centres in the United States and three in Italy.
The study was designed to randomise approximately 240 subjects to ensure that approximately 189 would complete six months of treatment. Eligible participants were healthy, premenopausal women aged 18 to 40 years, neither pregnant nor lactating, with a body mass index ≥ 18 kg/m2 and < 32 kg/m2. It was required that they should have had one spontaneous menstrual cycle with a duration of 23 to 35 days prior to the screening visit. Participants also agreed to use a nonhormonal, back-up method of contraception (e.g., condom, spermicidal foam, or contraceptive sponge) from the time of consent through the first seven days of study medication use and in situations where two or more tablets in a row were missed. The use of a nonhormonal method of contraception was also required in conjunction with the COC during and seven days after the use of drugs known to interact with COCs. Exclusion criteria included conditions contraindicating the use of COCs; the concomitant use of sex steroids (other hormonal medications, such as L-thyroxine, were allowed); a personal history of or current deep vein thrombosis (DVT), pulmonary embolism, or arterial thromboembolic disease; a genetically determined thrombophilia (including Factor V Leiden mutation, prothrombin mutation, protein C deficiency, protein S deficiency, or antithrombin III deficiency); a family history of venous thromboembolic event at age 40 or younger in a parent or sibling; and a history of abortion or delivery less than six months before screening.
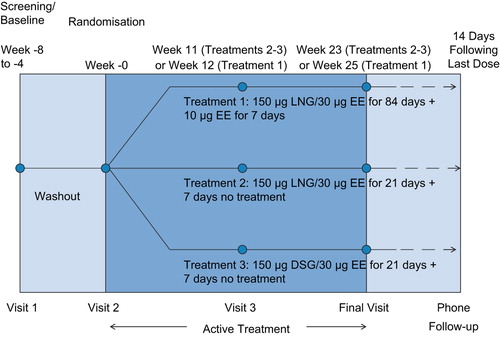
Subjects who met the eligibility criteria at the screening visit underwent baseline laboratory evaluations. With the exception of urine pregnancy tests, all clinical laboratory evaluations (blood tests, urinalysis, and Pap smear) were performed by ACM Global Central Laboratory (ACM), the central laboratory. ACM provided each site with a copy of the laboratory normal ranges for the study. Those who continued to meet eligibility criteria at the randomisation visit (Visit 2) were then randomly allocated in a 1:1:1 ratio to one of the three open-label treatment groups: the 91-day LNG/EE regimen previously defined (Treatment 1) and two traditional 21/7-day regimen COCs, namely, 21 days’ 150 μg LNG/30 μg EE followed by seven days of no treatment (Treatment 2) and 21 days’ 150 μg DSG/30 μg EE followed by seven days of no treatment (Treatment 3). In each of the three groups treatment was to last approximately six months. The overall duration of participation for all subjects was approximately eight months, which included a four-to-eight week screening/washout period, four study visits, and a two-week follow-up period ().
Participants randomised to Treatment 1 took the first dose on the Sunday following the first day of their menstrual bleeding after the randomisation visit and continued taking one tablet daily at approximately the same time for 26 consecutive weeks. Subjects assigned to Treatment 2 or Treatment 3 started taking the COC on the first day of their menses following the randomisation visit and continued to take it daily at approximately the same time for the first 21 days of each 28-day cycle.
Study endpoints and statistical methods
The primary study endpoint was the change from baseline in prothrombin F1 + 2 levels over the six-month treatment period for the per-protocol (PP) population. The PP population included all participants who had both a baseline and at least one post-baseline measurement of F1 + 2 obtained prior to major protocol violations, if any. Changes from baseline in prothrombin F1 + 2 levels were analysed using a repeated measures analysis of covariance (ANCOVA) with covariate adjustment for baseline, treatment, month, and the treatment by month interaction. Estimated treatment differences and the corresponding 95% confidence intervals (CIs) were calculated. A conclusion of noninferiority would be reached if the lower limit of the CI for a comparison of interest was greater than − 130 pmol/L.
Secondary endpoints were changes in D-dimer, activated partial thromboplastin time (APTT), endogenous thrombin potential (ETP)-based activated protein C (APC) resistance, factor II, factor VII, factor VIII, antithrombin, protein C, free and total protein S, total cortisol, and corticosteroid-binding globulin (CBG) over the six-month treatment period.
Safety endpoints included adverse events (AEs), clinical laboratory tests, and vital signs. AEs were recorded during the study through spontaneous reports by participants or noted by investigators at regularly scheduled visits.
RESULTS
Baseline demographic characteristics
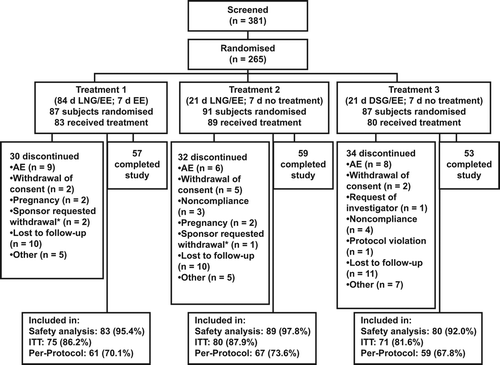
Of the 381 women screened, 265 met the inclusion criteria. Of these, 252 took at least one dose of study medication, and 169 completed the study (). The sponsor requested that three women be withdrawn from the study: one on Treatment 1 who had participated in another study funded by the sponsor; one on Treatment 1 who had elevated D-dimer at the randomisation visit; and one on Treatment 2 who exhibited a prothrombin alteration. Four women discontinued their participation after they became pregnant (two on Treatment 1 and two on Treatment 2). The PP population (principal analysis cohort for the primary endpoint) consisted of 187 treated subjects.
Figure 2 Subject disposition. *Sponsor requested withdrawal of one woman assigned to Treatment 1 who had participated in another study funded by the sponsor; one woman assigned to Treatment 1 who had elevated D-dimer at the randomisation visit; and one woman assigned to Treatment 2 who exhibited a prothrombin alteration. d, day; DSG, desogestrel; EE, ethinylestradiol; LNG, levonorgestrel; ITT, intent to treat.
There were no major differences in the demographic characteristics between the three treatment groups other than race ().
Table 1 Demographics and baseline characteristics of the per-protocol population (N = 187).
At baseline, the mean F1 + 2 levels were within normal range (41–372 pmol/L) for all three treatment groups, although the level was higher in subjects receiving Treatment 2 (207.2 pmol/L) than in those allocated to either Treatment 1 (141.0 pmol/L) or Treatment 3 (147.0 pmol/L), as shown in .
Primary endpoint
Each of the three COCs induced an increase in F1 + 2 levels () over the six-month study period. Changes from baseline in F1 + 2 were moderate and comparable for Treatment 1 (least squares [LS] mean change: 170 pmol/L) and Treatment 2 (LS mean change: 158 pmol/L), but markedly larger for Treatment 3 (LS mean change: 592 pmol/L).
Figure 3 Change from baseline in F1 + 2* at three and six months: per-protocol population. *Reference range for F1 + 2: 41–372 pmol/L. d, day; DSG, desogestrel; EE, ethinylestradiol; LNG, levonorgestrel; LS, least squares.
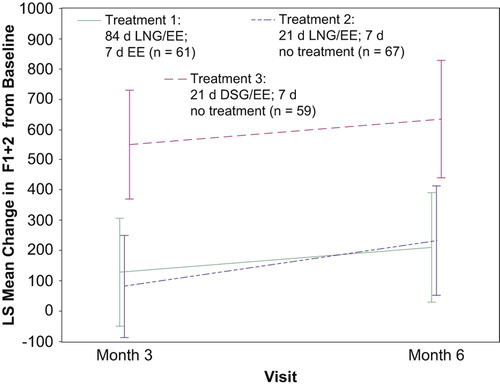
Noninferiority of Treatment 1 to Treatment 3 was demonstrated since the lower limit of the 2-sided 95% CI (− 18.3 pmol/L) was greater than the predefined noninferiority bound of − 130 pmol/L. The noninferiority of Treatment 1 to Treatment 2 was not demonstrated (lower limit of 95% CI: − 440 pmol/L), in fact, Treatments 1 and 2 were quite similar in terms of the LS mean change from baseline.
Secondary endpoints
Secondary endpoints evaluated the haemostatic parameters recommended in the European Medicines Agency's (EMA) Guideline on Clinical Investigation of Steroid Contraceptives in Women for assessing the risk of VTE in women using hormonal contraceptivesCitation16. Treatments 1 and 2 often elicited comparable effects. The response to Treatment 3 at times was the opposite of or more pronounced than the response to Treatment 1 ().
Table 2 Summary of secondary endpoints: Mean change from baseline in absolute values for selected haemostatic parameters.
For example, the increase in D-dimer, factor VII, and ETP-based APC resistance, was much greater with DSG/EE than with either LNG/EE regimen. Conversely, the levels of free protein S, total protein S, and APTT-based APC resistance were more markedly reduced by DSG/EE than by the LNG/EE regimens.
Whereas sex hormone-binding globulin (SHBG) levels were elevated in all three groups during the study, increases with Treatments 1 and 2 were similar and lower than those with Treatment 3 (). All regimens were associated with similar increases in total cortisol (LS mean changes of 217.9 nmol/L with Treatment 1, 262.4 nmol/L with Treatment 2, and 227.7 nmol/L with Treatment 3; p = not significant [NS] for each comparison) and cortisol-binding globulin (CBG; LS mean changes of 576.3 nmol/L with Treatment 1, 494.3 nmol/L with Treatment 2, and 596.8 nmol/L with Treatment 3; p = NS for each comparison).
Figure 4 Least squares (LS) mean change from baseline in sex hormone-binding globulin (SHBG; mIU/L): per-protocol population. The overall LS mean changes (± standard error [SE]) in SHBG (nmol/L) from baseline. Treatment 1 and Treatment 2 induced similar changes, 34.87 (± 8.40) and 30.85 (± 8.08) nmol/L, respectively. For Treatment 3, the LS mean change (± SE) showed a much greater elevation of 165.01 (± 8.67) nmol/L (p < 0.001 for Treatment 1 vs. 3). d, day; DSG, desogestrel; EE, ethinylestradiol; LNG, levonorgestrel.
![Figure 4 Least squares (LS) mean change from baseline in sex hormone-binding globulin (SHBG; mIU/L): per-protocol population. The overall LS mean changes (± standard error [SE]) in SHBG (nmol/L) from baseline. Treatment 1 and Treatment 2 induced similar changes, 34.87 (± 8.40) and 30.85 (± 8.08) nmol/L, respectively. For Treatment 3, the LS mean change (± SE) showed a much greater elevation of 165.01 (± 8.67) nmol/L (p < 0.001 for Treatment 1 vs. 3). d, day; DSG, desogestrel; EE, ethinylestradiol; LNG, levonorgestrel.](/cms/asset/83f3992c-1590-4588-b4a5-599f0873bd2c/iejc_a_918596_f0004_b.jpg)
Safety
Of the 252 subjects included in the safety analysis, 98 (39%) experienced at least one treatment-emergent AE (TEAE). The most commonly reported TEAEs were nausea (7%) and metrorrhagia (6%). Treatment 1 was shown to be safe and well tolerated in this study; there were no deaths, no other serious AEs, and no reports of safety concerns. The most commonly reported TEAE in subjects assigned to Treatment 1 was metrorrhagia (13%). Clinical and laboratory AEs observed were consistent with the expected safety profile of the 91-day LNG/EE regimen and those known to be associated with use of other COCs. None of the subjects in any group experienced a VTE.
DISCUSSION
Findings and interpretation
This study compared the effects of a 91-day LNG/EE extended regimen (Treatment 1: 84 days’ 150 μg LNG/30 μg EE; seven days’ 10 μg EE), a traditional 21/7 LNG/EE regimen (Treatment 2: 21 days’ 150 μg LNG/30 μg EE; seven days’ no treatment), and a traditional 21/7 DSG/EE regimen (Treatment 3: 21 days’ 150 μg DSG/30 μg EE for 21 days; seven days’ no treatment) on several coagulation factors and markers of thrombin formation over six months. Although an increase in F1 + 2 levels was observed for all three regimens, the change from baseline was considerably larger for the DSG-containing regimen. The haemostatic changes induced by the 91-day LNG/EE regimen (Treatment 1) and the traditional DSG- containing regimen (Treatment 3) were notably different. In contrast, the changes induced by Treatment 1 and the traditional Treatment 2, both of which included LNG/EE, were generally similar. In addition, SHBG levels were elevated in all three groups, although a much greater increase was observed with Treatment 3 (the DSG-containing regimen) compared with the other two groups ().
Minor effects on adrenal corticosteroids have been described with COCsCitation17. Because the EMA's Guideline on clinical investigation of steroid contraceptives in women recommends the assessment of these effectsCitation16, we have included measures of total cortisol and CBG as secondary endpoints in the present study. All three treatment modalities were associated with a rise in total cortisol and CBG levels but no between-group differences were observed.
Differences in results and conclusions in relation to other studies
COCs are known to affect certain haemostatic indicesCitation2,Citation18; however, it is unclear the extent to which these changes reflect the risk of VTE associated with COCs. Guidance provided in 2001 by the Committee for Property Medicinal Products of the EMA advised that although there was no urgency to modify the pattern of COC prescriptions, those containing LNG should be preferred over third-generation regimens for first time usersCitation19. A recent review by the EMA's Committee for Medicinal Products for Human Use also concluded that the absolute risk for VTE with low-dose combined hormonal contraceptives is small and the risk associated with these agents is lowest for regimens containing LNG, norethisterone, or norgestimateCitation20.
A very low incidence of VTE has previously been observed in clinical trials evaluating the 91-day LNG/EE regimen. No thromboembolic events were reported in a one-year study of 1006 women and a long-term extension study involving 320 womenCitation21,Citation22. Thus, the risk of VTE with extended regimen COCs containing LNG appears to be extremely low.
The findings from the current study are also consistent with previous research comparing traditional and extended COC regimens.Citation2 For example, Wiegratz et al.Citation2 measured haemostatic parameters in 59 women treated with either a traditional (21 + 7 days) regimen of 2 mg dienogest and 30 μg EE (DNG/EE) or a 91-day (84 + 7 days) extended regimen with the same daily doses of DNG/EE. The increase in F1 + 2 with the traditional regimen was slight whereas that associated with the extended regimen was somewhat – but not significantly – greater. Similarly, there were no significant between-group differences in the levels of fibrinogen, FVII, and D-Dimer after three and 12 months of treatmentCitation2. Overall, the changes in the various haemostasis variables observed during treatment with the extended DNG/EE regimen corresponded to those reported for traditional 21/7 COCsCitation2.
Among the secondary endpoints in our study, changes in SHBG levels with COCs are of particular interest given that they can be used to measure total oestrogenicity, which may predict VTE riskCitation23. Odlind and colleagues analysed data retrieved from a non-systematic literature review plus data from EMA application files to investigate the relationship between the risk of VTE with COCs and their impact on SHBG levelsCitation23. In their study, VTE risk was estimated using an EMA assessment of VTE risk in COC users, which was based on an expert review of published reports. Plotting VTE risk versus the mean SHBG increase observed with specific preparations, the authors observed a relationship between VTE risk and a rise in SHBG levels, and concluded that plasma SHBG levels may be a surrogate marker for VTE risk in users of COCs. Their analysis also revealed that increases in SHBG vary depending on the studies, and, as in our trial, the COC preparations evaluated. Monophasic preparations containing LNG, which were associated with the lowest risk of VTE, caused an average rise in SHBG of around 50%. However, COCs containing other progestins produced much greater increases. The average increase in SHBG associated with COCs was 200–300% for those containing DSG or gestodene, 150% for those including norgestimate, 250–300% for those containing drospirenone or DNG, and 300–400% for cyproterone acetate-based COCsCitation23. Although changes in SHBG levels in our study were similar between cyclic and extended LNG/EE regimens, a study comparing continuous 90 μg LNG/20 μg EE versus cyclic 100 μg LNG/20 μg EE reported greater SHBG rises with continuous LNG/EE than with cyclic LNG/EECitation24. The authors hypothesised that the greater changes in SHBG with the continuous regimen could possibly be explained by the lower total daily dose of LNG.
Strengths and weaknesses of the study
Strengths of our study included its multinational, multicentre, and randomised design and the racial diversity of the study population. In addition, we evaluated a large number of haemostatic and hormonal biomarkers – more than those recommended by the EMA – to thoroughly investigate the potential effects of these COCs on coagulation, fibrinolysis, and related VTE risk.
Limitations of the study included a lack of a placebo arm as well as not having a fourth-generation progestin comparator. Moreover, surrogate metabolic markers, such as SHBG, have not yet been definitely established as predictors of thrombosis in women treated with COCs. Consequently, large controlled trials with VTE as an endpoint are needed to objectively determine the clinical relevance of the associations we observed.
Relevance of the findings: Implications for clinicians and policymakers
Our findings confirm that the effects of COCs on haemostatic biomarkers vary by progestin, with LNG-based COCs causing a less pronounced effect than DSG-based COCs. COC cycle length appears to have little impact on haemostatic biomarkers. Clinicians should consider these findings when prescribing traditional or extended COC regimens for their patients.
Unanswered questions and future research
The haemostatic biomarkers evaluated in our study are generally thought to be useful surrogate markers of the risk of VTE and other thrombotic events associated with COCs. Nonetheless, these surrogate parameters have not been proven to capture the modifying effect of COCs on thrombotic risk. Additional research is needed to demonstrate the ability of these biomarkers to predict clinical outcomes and to clarify the comparative thrombotic risk of COCs, preferably through large controlled trials.
CONCLUSIONS
Although the clinical relevance is unclear, the LNG/EE-containing COCs (Treatments 1 and 2) had less impact on F1 + 2 levels than the DSG/EE-based regimens (Treatment 3) over the six-month treatment period. The 91-day and the 21/7-day LNG/EE regimens had similar effects on F1 + 2 levels. Changes in other haemostatic markers with the 84/7 and 21/7 LNG/EE regimens were similar but were less pronounced than those associated with the DSG-based COC. Thus, the results of this study would suggest that VTE risk for the extended 91-day regimen of LNG/EE, as evaluated by a set of haemostatic markers, is similar to that of the traditional 21/7-day LNG/EE COCs. However, additional studies assessing VTE as an endpoint are needed to confirm the results of our laboratory investigations.
ACKNOWLEDGEMENTS
Nicole Cooper of MedVal Scientific Information Services, LLC, provided medical writing and editorial assistance. This manuscript was prepared according to the International Society for Medical Publication Professionals’ ‘Good Publication Practice for Communicating Company-Sponsored Medical Research: the GPP2 Guidelines’ and the International Committee of Medical Journal Editors’ ‘Uniform Requirements for Manuscripts Submitted to Biomedical Journals’. Funding to support the preparation of this manuscript was provided to MedVal Scientific Information Services, LLC, Skillman, NJ by Teva Women's Health, Inc.
Funding: This study was sponsored by Teva Branded Pharmaceutical Products, R&D, Inc.
Declaration of interest: Dr Nappi: lecturer, advisory board member, and consultant for Bayer-Schering Pharma, Eli Lilly, Gedeon Richter, HRA Pharma, Merck Sharpe & Dohme, Novo Nordisk, Pfizer Inc, Shionogi, Teva/Theramex; Dr Paoletti: advisory board member for Bayer, Teva/Theramex, Gedeon Richter; Dr Howard and Dr Weiss are employees of Teva Global Medical Affairs; Ms Ricciotti is an employee of Teva Branded Pharmaceutical Products, R&D, Inc. The authors alone are responsible for the content and the writing of the paper.
REFERENCES
- Tans G, Curvers J, Middeldorp S, et al. A randomized cross-over study on the effects of levonorgestrel- and desogestrel-containing oral contraceptives on the anticoagulant pathways. Thromb Haemost 2000;84:15–21.
- Wiegratz I, Stahlberg S, Manthey T, et al. Effects of conventional or extended-cycle regimen of an oral contraceptive containing 30 mcg ethinylestradiol and 2 mg dienogest on various hemostasis parameters. Contraception 2008;78:384–91.
- Lidegaard Ø, Nielsen LH, Skovlund CW, et al. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ 2011; 343:d6423.
- van Hylckama Vlieg A, Helmerhorst FM, Vandenbroucke JP, et al. The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: Results of the MEGA case-control study. BMJ 2009;339:b2921.
- Martínez F, Ramírez I, Pérez-Campos E, et al. Venous and pulmonary thromboembolism and combined hormonal contraceptives. Systematic review and meta-analysis. Eur J Contracept Reprod Health Care 2012;17:7–29.
- Wiegratz I, Kuhl H. Metabolic and clinical effects of progestogens. Eur J Contracept Reprod Health Care 2006; 11:153–61.
- Tchaikovski SN, Rosing J. Mechanisms of estrogen-induced venous thromboembolism. Thromb Res 2010;126:5–11.
- van Vliet HA, Bertina RM, Dahm AE, et al. Different effects of oral contraceptives containing different progestogens on protein S and tissue factor pathway inhibitor. J Thromb Haemost 2008;6:346–51.
- WHO Task Force on Oral Contraceptives. A multicentre study of coagulation and haemostatic variables during oral contraception: variations with four formulations. Task Force on Oral Contraceptives – WHO Special Programme of Research, Development and Research Training in Human Reproduction, World Health Organization, Geneva, Switzerland. Br J Obstet Gynaecol 1991;98:1117–28.
- Middeldorp S, Meijers JC, van den Ende AE, et al. Effects on coagulation of levonorgestrel- and desogestrel-containing low dose oral contraceptives: A cross-over study. Thromb Haemost 2000;84:4–8.
- Lidegaard Ø, Løkkegaard E, Svendsen AL, Agger C. Hormonal contraception and risk of venous thromboembolism: National follow-up study. BMJ 2009;339:b2890.
- Hannaford PC. Epidemiology of the contraceptive pill and venous thromboembolism. Thromb Res 2011; 127(Suppl. 3):S30–4.
- Edelman A, Lew R, Cwiak C, et al. Acceptability of contraceptive-induced amenorrhea in a racially diverse group of US women. Contraception 2007;75:450–3.
- Sulak PJ, Carl J, Gopalakrishnan I, et al. Outcomes of extended oral contraceptive regimens with a shortened hormone-free interval to manage breakthrough bleeding. Contraception 2004;70:281–7.
- Kaunitz AM, Portman DJ, Hait H, Reape KZ. Adding low-dose estrogen to the hormone-free interval: Impact on bleeding patterns in users of a 91-day extended regimen oral contraceptive. Contraception 2009;79:350–5.
- European Medicines Agency and Committee for Medical Products for Human Use. Guideline on clinical investigation of steroid contraceptives in women. Accessed 24 March 2014 from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003349.pdf
- Wiegratz I, Kutschera E, Lee JH, et al. Effect of four oral contraceptives on thyroid hormones, adrenal and blood pressure parameters. Contraception 2003;67:361–6.
- Oral Contraceptive and Hemostasis Study Group. The effects of seven monophasic oral contraceptive regimens on hemostatic variables: Conclusions from a large randomized multicenter study. Contraception 2003; 67:173–85.
- Garattini S, Bertele V. Adjusting Europe's drug regulation to public health needs. Lancet 2001;358:64–7.
- European Medicines Agency. Benefits of combined hormonal contraceptives (CHCs) continue to outweigh risks – CHMP endorses PRAC recommendation. Accessed 24 March 2014 from: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2013/11/WC500155455.pdf
- Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception 2006;73:229–34.
- Davis M. Evaluation of the long-term safety of a 91-day extended regimen oral contraceptive with low-dose estrogen in place of placebo. Contraception 2007;76: 172(abstract P42).
- Odlind V, Milsom I, Persson I, Victor A. Can changes in sex hormone binding globulin predict the risk of venous thromboembolism with combined oral contraceptive pills?Acta Obstet Gynecol Scand 2002; 81:482–90.
- Rad M, Kluft C, de Kam ML, et al. Metabolic profile of a continuous versus a cyclic low-dose combined oral contraceptive after one year of use. Eur J Contracept Reprod Health Care 2011;16:85–94.