ABSTRACT
Objectives: The clinical utility of extended regimen combined oral contraceptives (COCs) is increasingly being recognised. Our objective was to understand the attitudes of women and clinicians about the use of these regimens. We present the rationale for extended regimen COCs from a historical perspective, and trace their evolution and growing popularity in light of their clinical benefits. We conclude by offering potential strategies for counselling women about extended regimen COC options.
Methods: We conducted a MEDLINE search to identify and summarise studies of extended regimen COCs, focusing on attitudes of women and clinicians regarding efficacy, safety/tolerability and fewer scheduled bleeding episodes and other potential benefits.
Results: The body of contemporary literature on extended regimen COCs suggests that their contraceptive efficacy is comparable to that of conventional 28-day (i.e., 21/7) regimens. For women seeking contraception that allows infrequent scheduled bleeding episodes, particularly those who suffer from hormone withdrawal symptoms and cyclical symptoms (e.g., headache, mood changes, dysmenorrhoea, heavy menstrual bleeding), extended regimen COCs are an effective and safe option. Although satisfaction with extended regimen COCs in clinical trials is high, misperceptions about continuous hormone use may still limit the widespread acceptance of this approach.
Conclusions: Despite the widespread acceptance among clinicians of extended regimen COCs as an effective and safe contraceptive option, these regimens are underused, likely due to a lack of awareness about their availability and utility among women. Improved patient education and counselling regarding the safety and benefits of extended regimen COCs may help women make more informed contraceptive choices.
Introduction
Combined oral contraceptives (COCs) have undergone numerous changes since they were introduced over 50 years ago. Early COCs consisted of 28-day pill packs, typically known as 21/7 COC regimensCitation1. Such regimens, still in widespread use, provide 21 days of active combination hormone pills followed by a hormone-free interval (HFI) of seven placebo pillsCitation2,Citation3. The aim of these regimens is to induce withdrawal bleeding every 28 days, which historically has served to mimic a monthly menstrual cycle and reassure the user that she is not pregnantCitation1–3. Several variations of the original 21/7 regimen, which maintain a 28-day cycle but have fewer hormone-free days (i.e., 24/4 and 26/2), are now available and offer the benefits of shorter, lighter withdrawal bleedsCitation4–6. It is recognised, however, that monthly withdrawal bleeds in any form are unnecessary and often inconvenient. Moreover, hormone withdrawal during the HFI may increase the risk of escape ovulation and induce a variety of symptoms, such as headaches, bloating and pelvic painCitation1,Citation7–11.
One strategy to minimise withdrawal bleeding and maintain ovarian suppression has been to extend the COC cycle beyond 28 days. In this increasingly popular strategy, the number of active pills administered per cycle and the amount of time between HFIs is increasedCitation12. By extending the time between scheduled bleeding episodes, an important objective is achieved: namely, the total number of scheduled bleeding episodes is reducedCitation13. Extended regimen COCs may reduce the interference of scheduled bleeding with daily activities, such as sexual activity, exercise, sports and work (including menstruation-related absence from work) and reduce the costs and inconvenience associated with feminine hygiene productsCitation14–18.
In addition to allow women the option to avoid menstruation, extended, flexible-extended and continuous regimens have become recognised in recent years as useful approaches to treat endometriosis, dysmenorrhoea and menstrual-related symptomsCitation16,Citation19–23. Given these various positive attributes, one might possibly expect the adoption of extended regimen COCs to be nearly universal; however, there is evidence to suggest that such regimens are underusedCitation24–26. We were interested, therefore, to understand whether attitudes or perceptions of women and clinicians constituted barriers to the use of extended regimen COCs.
The objective of this review is to summarise the available information on: (1) the evolution and rationale for extended regimen COCs, considering their efficacy, safety and clinical utility; (2) women’s and clinicians’ attitudes towards extended regimen COCs; and (3) strategies for improving communication about COC options to increase awareness among clinicians and women about the potential benefits of extended regimen COCs.
Methods
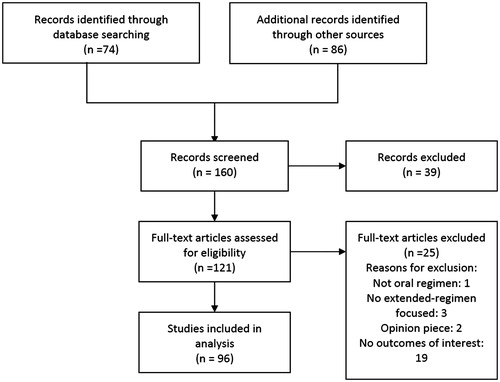
Focusing on women’s and clinicians’ attitudes and usage patterns related to extended or flexible regimen COCs, we collected relevant information for our review by performing a MEDLINE search of articles published in English between 1970 and 2014. We used the following search strategy: oral AND (contraception OR contraceptives) AND (extended regimens OR flexible regimens) AND (attitudes OR satisfaction OR adherence OR efficacy OR compliance OR counselling OR education). Of the 160 articles screened, 96 were included in the analysis (). Reference lists of the retrieved articles were also reviewed to identify references not found using electronic search methods.
Although there is no universally accepted definition of what constitutes an extended COC regimen, we defined such regimens for the purpose of this review as those that include more than 28 days’ COC pills followed by 7 or fewer days of no pills, placebo or low-dose ethinylestradiol (EE). We also included flexible-extended regimens, whereby users take COCs continuously until they experience persistent unscheduled bleeding, and then begin an HFI of 7 or fewer daysCitation27. These extended and flexible-extended regimens differ from continuous regimens, which provide uninterrupted COCs for 1 year or more without an HFICitation2,Citation27 and are not the focus of this article. Furthermore, we evaluated review articles on these topics and their reference lists to identify additional relevant manuscripts.
Results and discussion
Rationale for extended regimen COCs: efficacy, safety and clinical utility
One of the most important and recognisable trends in the evolution of COCs has been the reduction in hormone dosages to lower the risk of thrombotic eventsCitation2. However, while the lower hormone dosages in today’s COCs are associated with a lower risk of thrombotic events, they also have the potential to increase the risk of follicular activity and escape ovulation, particularly during the traditional 7-day HFICitation9,Citation28. In fact, follicular growth taking place during the 7-day HFI resembles that seen during the early follicular phase of a spontaneous menstrual cycleCitation29,Citation30.
The limitations of the 7-day HFI prompted development of various regimens, including those with a shortened HFI (i.e., 24/4 and 26/2 regimens), regimens that substitute low-dose EE for the HFI and extended regimen COCs, which are the focus of this review. Each of these strategies has been shown to provide greater pituitary–ovarian suppression, reduce follicular development and the risk of contraceptive failure and decrease the incidence of symptoms related to hormone withdrawalCitation9,Citation19,Citation31–33.
Evolution of extended regimen COCs
Extended regimen COCs have been evolving, influenced by many factors, including contraceptive efficacy, safety and bleeding patterns.
The first approval in the USA of an extended regimen COC was based on a large randomised controlled trial that demonstrated the efficacy of 84 days’ levonorgestrel (LNG) 150 μg/EE 30 μg followed by seven placebo daysCitation34. Pearl Indices, based on method failure rates, were 0.60 and 1.78 for the 84/7 and the 21/7 regimen, respectivelyCitation34. Compared with the 21/7 regimen, the 84/7 regimen was associated with significantly fewer total days of scheduled bleeding/spotting; however, an increased incidence of unscheduled breakthrough bleeding/spotting was reportedCitation34, thus revealing an important limitation of extended regimen COCs compared with traditional 21/7 regimensCitation34.
Since unscheduled breakthrough bleeding may lead to poor adherence that can ultimately affect contraceptive efficacyCitation13, modifications to the original 84/7 regimen were introduced. One such modification was to substitute low-dose EE (10 μg/day) for placebo or no treatment during the traditional HFICitation35,Citation36. This strategy has been associated with increased ovarian suppression, including a potentially reduced risk of escape ovulation and unscheduled breakthrough bleedingCitation9,Citation13. With regard to bleeding patterns, a cross-study comparison of 84/7 regimens reported less scheduled bleeding and quicker reduction in the incidence of unscheduled bleeding when low-dose EE was substituted for the traditional 7-day HFICitation13. Since then, an even lower continuous EE dose 84/7 regimen (84 days’ LNG 100 μg/EE 20 μg plus 7 days’ EE 10 μg) has been introducedCitation37,Citation38.
Higher EE dosages in COC regimens appear to provide greater endometrial stabilisation and less breakthrough bleedingCitation39,Citation40. A third type of 84/7 regimen was developed that incorporates ascending EE dosages during the first 84 days with constant LNG dosages, followed by 7 days’ low-dose EE. The timing of increased EE dosages in the pill pack is intended to coincide with the time that unscheduled bleeding with extended regimen COCs typically occurs (both in clinical practice and clinical trials)Citation36,Citation40. This ascending dose, extended regimen of 42 days’ LNG 150 μg/EE 20 μg, 21 days’ LNG 150 μg/EE 25 μg and 21 days’ LNG 150 μg/EE 30 μg, followed by seven days’ EE 10 μg, was demonstrated to be effective in preventing pregnancyCitation36 and may be associated with less unscheduled bleeding compared with other LNG/EE extended regimensCitation36,Citation41. This new ascending dose, extended regimen was recently approved in the USA.
A final strategy to reduce unscheduled bleeding is to initiate a short HFI when persistent unscheduled bleeding occurs, a tactic known as a flexible-extended regimen. One randomised active-controlled study comparing a 24/4 regimen with two flexible-extended regimens found this to be an effective approachCitation27.
Efficacy
Two recent extensive systematic reviews of extended and continuous regimen COCs concluded that the risk of pregnancy did not differ between cyclical and extended regimensCitation16,Citation42. Observational data, however, suggest that regimens with shorter or fewer HFIs may be associated with reduced pregnancy ratesCitation43,Citation44. One analysis of a retrospective claims database revealed lower contraceptive failure rates with 84/7 regimens compared with 21/7 and 24/4 regimens. At 1 year, rates of pregnancy were significantly lower with 84/7 regimens vs. 21/7 regimens (4.4% vs. 7.3%; p < 0.0001) and with 84/7 regimens vs. 24/4 regimens (4.4% vs. 6.9%; p < 0.0001)Citation44.
Bleeding patterns
Since a major goal of extended regimen COCs is to reduce the incidence of scheduled withdrawal bleeds as well as overall bleeding, most studies evaluating the effectiveness of extended regimens have also included bleeding patterns as an outcomeCitation15,Citation27,Citation34–37,Citation45. Moreover, since ‘escape’ follicular development and bleeding patterns are often linked, the two systematic reviews mentioned previously also address bleeding outcomesCitation16,Citation42.
A recent Cochrane review by Edelman and colleaguesCitation16 concluded that most trials found either no difference or less bleeding and/or spotting with extended/continuous vs. cyclical regimens, although most users of extended regimen COCs will experience occasional unscheduled (breakthrough) bleeding or spotting.
Although most studies report an increased incidence of unscheduled bleeding with extended regimens during early cyclesCitation16,Citation42, it has also been consistently documented that the frequency and intensity of such bleeding decreases over timeCitation42. By the fourth extended cycle, the incidence of unscheduled bleeding is generally comparable to that seen among users of conventional cyclical regimensCitation34,Citation46.
Safety
Considering that some extended regimen COCs may provide a greater cumulative oestrogen dose compared with similarly dosed cyclical regimens, women and clinicians may be concerned that extended regimens may increase certain safety risks. Accumulating data, however, provide reassurance regarding the safety of long-term use of extended regimen COCsCitation16,Citation47–49. Current evidence suggests that adverse events associated with extended regimens are similar to those seen with 28-day cyclical regimens. There is also no evidence that the risk of stroke, myocardial infarction or thrombosis is increased with extended regimens compared with 28-day regimensCitation42.
Potential for fewer hormone withdrawal symptoms
Since the incidence of symptoms associated with hormone withdrawal and menstruation is related to the number of hormone withdrawal episodes, as the number of such episodes decreases so should their associated symptomsCitation16,Citation42. Extended regimens clearly improve dysmenorrhoea by decreasing the total number of withdrawal bleedsCitation42. In an early study of 84 days’ EE and lynestrenol and 7 days’ placebo, 20% of participating women had fewer menstrual symptoms compared with their previous pill regimenCitation50. These benefits are particularly apparent among women who experience premenstrual syndrome or symptoms associated with the HFI when using conventional regimensCitation42.
In a study of the use of extended regimens to delay withdrawal bleeding and reduce hormone withdrawal symptoms, 41–86% of women who chose extended regimen COCs experienced an improvement in their symptomsCitation51. Among the 59% of women who chose to continue using extended regimen COCs, 94% reported improvement in their quality of lifeCitation51.
In one study of adolescents who adhered to an extended regimen, the prevalence of dysmenorrhoea decreased by 56%, spending on painkillers decreased by 75% and absenteeism from work and school decreased by 92%Citation52.
Bleeding preferences
Many women would prefer to eliminate or reduce the frequency of scheduled bleeding if given the choice ()Citation25,Citation26. In one of the first studies to evaluate an 84/7 COC regimen, 82% of 196 participants welcomed having fewer withdrawal bleeds; both women and clinicians appreciated this and other aspects related to taking an extended regimen COCCitation50. Although the lack of scheduled bleeding was disturbing to some, many women and clinicians felt this regimen was easier to follow and was well accepted. In fact, 91% of women still using the extended regimen at the end of the study refused to revert to the standard monthly regimenCitation50.
Table 1. Attitudes of women towards extended regimen contraception: results of representative surveys of reproductive aged womenCitation25,Citation26,Citation61,Citation63,Citation67.
According to a 2007 Canadian consensus statement, extended regimens are also associated with a greater reduction in the use of hygiene products compared with conventional cyclesCitation42,Citation53. The lower use of these products and lower associated out-of-pocket expenses constitute yet another reason for women to prefer extended regimen COCs. Moreover, the increased number of tablets consumed does not appear to increase the overall costs of these regimensCitation42.
Reduced withdrawal bleeding may also reduce the risk of anaemia in women using extended COC regimens. A recent study indicated, however, that relatively few women are aware of this potential benefitCitation54.
Adherence and satisfaction
Missed pills, in the real world, particularly those missed during the first week of COC use, may increase the risk of follicular development and cause contraceptive failureCitation9,Citation10. It has been hypothesised that when extended regimen COCs are used to avoid menstruation for convenience or personal preference, they may lead to improved adherence and greater user satisfaction due to a reduction in the number of hormone withdrawal episodes and greater number of active pills availableCitation42,Citation46. In one survey of 617 gynaecologists following 3316 women throughout France, 23% of women using conventional COCs reported missing a pill at least once during a 28-day cycle, and 42% of women who missed a pill did so during the first week following the HFICitation55. This led the authors to conclude that, by reducing the opportunity to miss pills during the first week of the cycle, using continuous regimens might improve compliance. Despite this, a recent Cochrane analysis found no difference in compliance or adherence between users of extended or continuous regimen COCs when patient compliance was reportedCitation16.
User satisfaction could possibly be considered a proxy for user adherence. Indeed, high user satisfaction has been consistently reported with extended regimen COCsCitation16,Citation27,Citation34,Citation42,Citation56,Citation57, and this satisfaction typically increases as the number of bleeding episodes decreasesCitation58. By contrast, another study with a flexible-extended regimen COC reported lower satisfaction with continuous than with cyclical COCs, both with regard to satisfaction with bleeding outcomes and overall satisfactionCitation57. It is noteworthy, however, that survey data indicate that the majority of women using hormonal contraceptives would accept unpredictable bleeding initially if they had fewer withdrawal bleeds over timeCitation59.
In an early study of Dutch women’s preferences, 81% of those surveyed would prefer to modify their menstrual cycle (less painful, shorter or lighter periods). Most of these women preferred to have a bleeding frequency of less than once a month or neverCitation60. Approximately two-thirds of reproductive aged women surveyed in Germany indicated they would prefer to bleed less frequently than monthly, and 37–46% would prefer never to bleedCitation61. Among 350 Italian women of reproductive age, only 32% indicated a preference to bleed monthly, whereas 68% preferred to bleed less than once a monthCitation62. Among COC users, approximately 57% preferred to bleed less than once a month and 26% preferred to bleed every 3 monthsCitation62.
Contemporary Swiss women have similar attitudes towards menstrual bleeding. A recent study reported that 32% preferred to bleed every 2–6 months and 29% preferred not to bleed at allCitation63. Interestingly, preferences for bleeding frequency in this study did not appear to differ between women who experienced menstrual symptoms and those who did not. The findings of this survey suggested that predictability of bleeding may be more important for some women than the ability to postpone it. Indeed, more than 80% of women felt that the predictability of bleeding and avoidance of unscheduled bleeding were very importantCitation63.
A desire for less frequent bleeding is not limited to women who experience symptoms related to menstruation or withdrawal bleeding. In one study of 270 women without menstrual symptoms, 76% reported that menstrual periods interfered with their sexual life, 29% preferred not having their period at work and 48% felt that menstruation interfered with their sporting activitiesCitation18. If given the choice, more than half of the participants indicated that they would prefer less frequent periodsCitation18. Of these women who would prefer less frequent periods, half would prefer amenorrhoea. Importantly, 73% of the women who preferred a reduction in menstrual frequency said they would use a drug to accomplish thisCitation18.
Data indicate that many women have already used COCs to delay bleeding, suggesting that many would welcome a COC option that modified the frequency of their scheduled bleeding episodes. A 2008 survey reported that approximately one-third of Italian COC users had modified their COC regimen to delay the occurrence of withdrawal bleedingCitation62. A more recent survey of US university students found that 17% of respondents had altered their scheduled bleeding pattern by deviating from the instructions in the package insertCitation26. While women who preferred a structured schedule and consistent monthly cycles were less likely to manipulate the timing of their scheduled bleeding, women who were older, those who used oral (but not transdermal or vaginal) contraceptives and those who preferred to bleed less frequently were more likely to report manipulating their COC scheduleCitation26. Clinicians providing guidance regarding the off-label manipulation of 28-day COC formulations to approximate extended regimens should consider the possibility that this practice may result in misuse, potentially increasing the risk of contraceptive failure.
Awareness of and attitudes towards extended regimen COCs
Awareness and knowledge
Despite the consistent survey data demonstrating that the majority of reproductive aged women preferred to bleed less often than monthlyCitation25,Citation26,Citation56,Citation64, a US-based study found that 73% of women had never even heard of using COCs to manipulate their monthly bleeding episodesCitation25. Of concern, only half of the university students surveyed by Lakehomer et al.Citation26 in a US study reported learning about cycle manipulation from health care providers (HCPs). Another study of family planning clinics in London revealed that extended regimen COCs were discussed with as few as 6% of women attending centres that provided specialist contraceptive servicesCitation65. This finding is corroborated by previous research from Spain that demonstrated that 90% of women had never been offered the option to suppress monthly bleeding by their gynaecologistCitation66. A lack of discussion or recommendation for extended regimens by HCPs, particularly in certain European countries, may be explained, at least in part, by the lack of availability of approved extended regimen COCs.
In the US, where several extended regimen COCs are approved for use, it appears that only 2.5% of women prescribed COCs are prescribed an extended regimen COCCitation24. Although this figure may not represent their actual use, it is evident that many women interested in menstrual suppression are not offered extended regimen COCs by their HCPs.
Attitudes, beliefs and misconceptions
Attitudes and beliefs about menstruation among women around the world have been evolving. Although many women still believe that monthly bleeding is necessary, others would prefer to bleed less frequently or not at all ()Citation26,Citation67. These changing preferences have been observed in women from both developed and developing countries, and, as reviewed below, have been particularly well documented in studies of Western European womenCitation16,Citation25,Citation56,Citation67,Citation68. Because preferences regarding menstruation are related to cultural beliefs, they may vary from region to regionCitation69.
Even when women learn about how using extended regimens can reduce the frequency of bleeding, some may be concerned about this approach ()Citation50. For example, some may worry about the risk of ‘menstrual build-up,’ fear that skipping monthly bleeding may lead to an ‘unnatural state,’ or worry that each missed period represents a possible pregnancyCitation16. However, with reassurance regarding the safety of this approach, most women would prefer to delay or never have a periodCitation16,Citation60,Citation67,Citation68. Among gynaecologists practising in Brazil, 93% of those surveyed indicated that medically-induced amenorrhoea represents no risk to women’s health and 83% said they prescribed contraceptives to control menstruation or induce amenorrhoeaCitation70. Still, not all HCPs are convinced of the clinical utility of extended regimen COCs. In the study of family planning clinics in LondonCitation65, both HCPs and women expressed concerns about the risks of avoiding monthly bleeding, the impact of extended regimen COCs on future fertility and the clinical impact of breakthrough bleeding.
Return to fertility. Some women and clinicians have expressed concerns about resumption of cycles and fertility following the use of hormonal contraceptives that suppress menstruationCitation50,Citation54,Citation65. Although studies evaluating return to fertility following the discontinuation of extended regimen COCs are relatively uncommon, those that have assessed this outcome have reported that fertility returns promptly after their discontinuationCitation42,Citation71,Citation72. Among women discontinuing extended regimen COCs without starting other hormonal contraceptives, the median time to withdrawal bleeding was 32 days, with 77% of women returning to ovulatory capacity (defined as serum progesterone ≥15.9 nmol/L) within 32 days and 99% of women having spontaneous menstruation or pregnancy within 3 months of discontinuationCitation73.
Effects on the endometrium. Theoretical concerns about the potential effects on the endometrium of extended regimen COCs that contain more days of oestrogen-containing pills are common among clinicians but also among some women considering an extended regimen. Histological examinations of the endometrial lining have, however, confirmed an inactive endometrium, likely induced from adequate progestin exposure, in women using either cyclical or extended regimensCitation16,Citation74. These findings have been confirmed by multiple large trials of extended regimen COCs, demonstrating that long-term use of these regimens does not cause endometrial pathology but largely produces an atrophic endometriumCitation42,Citation46,Citation74. Similarly, an analysis of the endometrial effects of an extended LNG 150 μg/EE 30 μg + EE 10 μg COC found no evidence of endometrial hyperplasia and confirmed the endometrial safety of this regimenCitation75. Studies have also demonstrated a rapid return to a normal cycling endometrium histology after the discontinuation of extended regimen LNG/EE COCs.Citation74,Citation75
Current prescribing patterns and additional perspectives on extended regimen COCs
Recent evidence suggests that HCPs whose focus is women’s health are comfortable with prescribing extended regimen COCs; 70–92% of US clinicians have recommended them in their practicesCitation70,Citation76–79. Not surprisingly, gynaecologists are more likely to prescribe extended regimens compared with primary care physiciansCitation77. The most commonly prescribed extended regimen COC in the USA is an 84/7 formulation that incorporates a 7-day HFICitation76. The majority of general HCPs (>73%) continue to prescribe 28-day COCs as the most common regimenCitation76.
In the UK, family planning specialists appear to have been slower to adopt extended regimen COCs than those in other countries. A survey of clinicians at three family planning clinics in London revealed that only one of the three clinics initiated and maintained guidelines for the use of extended regimensCitation65. The frequency of counselling women about extended regimen COCs was highly variableCitation65. In the clinic that maintained guidelines, 60% of staff provided counselling on extended regimen COCs to more than 50% of womenCitation65. In the other two clinics, extended regimen COCs were discussed with only 6–20% of women. Approximately one-third of respondents felt more comfortable prescribing 21/7 regimens, due, in large part, to a lack of familiarity with other regimensCitation65. Respondents also expressed an interest in receiving more information on prescribing recommendations and on the long-term effects of extended regimen COC use. The results of this survey provide evidence that more training on the use of extended regimen COCs may be needed, even among family planning specialistsCitation65.
Extended regimen COCs seem to be more frequently prescribed in other areas of Europe and in South America. Among female gynaecologists practising in Germany and Austria, 97% had prescribed extended regimen COCs at least onceCitation80. One Brazilian survey of 1097 gynaecologists found that 93% of women seen in their practices had requested extended or continuous regimen COCs; 94% of those surveyed had already prescribed them at least onceCitation81. Interestingly, the female gynaecologists surveyed were more likely to prescribe extended regimen COCs, while the male gynaecologists preferred prescribing continuous regimensCitation81. A second study confirmed that Brazilian gynaecologists were favourably inclined towards prescribing these regimens for control of menstrual bleeding or to induce amenorrhoeaCitation70.
Users of extended regimen COCs do not appear to have an increased risk of breast cancer, infertility or thrombosis beyond that of conventional 28-day regimensCitation76. Attitudes and perceptions of surveyed HCPs regarding the use of extended regimen COCs revealed that 82% of the participants did not believe that they increased health risks. Much smaller percentages believed they increased the risk of breast cancer (8%), infertility (4%) or thrombosis (14%) compared with 28-day regimensCitation76. Among German and Austrian gynaecologists, only 3% expressed concerns related to these agents’ effects on the breast or fertility, or other adverse eventsCitation80.
Perhaps the greatest endorsement of the safety and utility of extended regimen COCs comes from female gynaecologists’ widespread personal contraceptive choicesCitation82. Almost all physicians interviewed for one survey reported using hormonal contraceptives to control their own bleeding or that of their partnerCitation70. Such data are particularly important considering that personal contraceptive use may affect HCPs’ own prescribing and counselling practiceCitation83.
Strategies for improving communication about extended regimen COCs
Concerns regarding the use of extended regimen COCs can likely be addressed through effective counselling. Research has shown that clinicians who use a structured, patient-centred approach to contraceptive counselling that includes shared decision-making may influence contraceptive use, and it is an approach preferred by womenCitation84–87. Shared decision-making represents an approach that considers patient preferences and respects patient autonomy, using a structure that enables clinicians to emphasise highly effective contraceptives while considering the woman’s preferencesCitation84. Preliminary data suggest that approaches involving the tenets of shared decision-making may increase the use of effective contraceptionCitation85, but data on their impact on extended regimen COC use is lacking. Studies of interventions designed to improve the delivery of information about contraception have been limited and mostly unsuccessfulCitation84,Citation88, but several best practices have been proposed for general contraceptive counselling.
Women should, first and foremost, understand that the use of an extended regimen COC to delay or eliminate scheduled bleeding episodes is not harmful to their healthCitation42,Citation46–49,Citation89–91 (Citation92–95). Clinicians should also ensure that they provide information about the side effects of the contraceptives considered and communicate about these side effects in a way that is meaningful to womenCitation84. Issues to discuss include the long-term safety of extended regimensCitation47–49, the likelihood of unscheduled bleeding in early cyclesCitation16,Citation42, the rapid return to fertility once extended regimen COCs are discontinuedCitation42,Citation71 and other potential benefits of extended regimen COCsCitation16,Citation42,Citation72. Given the high rate of discontinuation of COCs, proactively addressing possible logistical, financial or medical problems that may arise and providing recommendations for addressing these problems may ultimately improve the use of extended regimen COCsCitation84.
Table 2. Key points for counselling women considering extended regimen COCsCitation92–95.
Information on various COC regimens and options should be presented to meet each woman’s individual needs. Such targeted counselling is particularly important given the evidence suggesting that the quality of physician–patient communication may help ensure patient adherence and patient satisfactionCitation24,Citation70,Citation78,Citation96.
By considering women’s personal preferences regarding menstruation and scheduled bleeding, clinicians can individualise COC regimens to best meet their needsCitation42. For example, it may be reassuring to women with a higher body mass index that studies of the safety and efficacy of extended regimen COCs have included such womenCitation35,Citation36.
Despite considerable evidence supporting the efficacy and safety of extended regimen COCs, some clinicians may benefit from targeted training regarding these regimens to help expand their patients’ choices and increase the acceptance of extended regimens around the worldCitation65.
Conclusion
A considerable body of evidence supports the efficacy, safety, convenience and clinical benefits of extended regimen COCs. Many women, however, lack awareness of the availability or utility of extended regimens. Other women may have misperceptions regarding the need for monthly bleeding or the safety of extended regimens. Consequently, women who wish to use COCs should be offered the opportunity to choose the frequency of their withdrawal bleeding. Increased awareness and empowerment of women through patient-centred counselling may help meet the needs of those desiring effective contraception with fewer monthly bleeds.
Acknowledgements
The authors thank Nicole Cooper of MedVal Scientific Information Services, LLC, for providing medical writing and editorial assistance. The authors also thank Dr David Portman for his review and input during the development of this manuscript. This manuscript was prepared according to the International Society for Medical Publication Professionals’ ‘Good Publication Practice for Communicating Company-Sponsored Medical Research: the GPP2 Guidelines’ and the International Committee of Medical Journal Editors’ ‘Uniform Requirements for Manuscripts Submitted to Biomedical Journals.’
Financial disclosures
REN has served as a consultant, lecturer and/or member of an advisory board for Bayer Pharma, Eli Lilly, Gedeon Richter, HRA Pharma, MSD, Novo Nordisk, Pfizer Inc., Shionogi and Teva.
AMK serves as a member of advisory boards for Actavis, Bayer, Merck and Teva. His institution (University of Florida) receives research support from Agile, Bayer and Merck.
JB has received research grants from MSD and Procter and Gamble, served on advisory boards for Abbott, Actavis, Bayer, MSD and Pfizer and served on speaker’s bureaus for Abbott, Actavis, Bayer, Exeltis, MSD and Pfizer.
Declaration of interest
Medical writing assistance was provided by MedVal Scientific Information Services, LLC (Skillman, NJ, USA) and was funded by Teva Branded Pharmaceutical Products R&D, Inc. (Frazer, PA, USA). Teva provided a full review of the article.
REFERENCES
- Thomas SL, Ellertson C. Nuisance or natural and healthy: should monthly menstruation be optional for women? Lancet 2000;355:922–4.
- Burkman R, Bell C, Serfaty D. The evolution of combined oral contraception: improving the risk-to-benefit ratio. Contraception 2011;84:19–34.
- Pincus G. Control of conception by hormonal steroids. Science 1966;153:493–500.
- Mansour D, Verhoeven C, Sommer W, et al. Efficacy and tolerability of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β-oestradiol in a 24/4 regimen, in comparison to an oral contraceptive containing ethinylestradiol and drospirenone in a 21/7 regimen. Eur J Contracept Reprod Health Care 2011;16:430–43.
- Kaunitz AM, Burkman RT, Fisher AC, et al. Cycle control with a 21-day compared with a 24-day oral contraceptive pill: a randomized controlled trial. Obstet Gynecol 2009;114:1205–12.
- Ahrendt HJ, Makalova D, Parke S, et al. Bleeding pattern and cycle control with an estradiol-based oral contraceptive: a seven-cycle, randomized comparative trial of estradiol valerate/dienogest and ethinyl estradiol/levonorgestrel. Contraception 2009;80:436–44.
- Bitzer J. Hormone withdrawal-associated symptoms: overlooked and under-explored. Gynecol Endocrinol 2013;29:530–5.
- Kaunitz AM. Menstruation: choosing whether…and when. Contraception 2000;62:277–84.
- Vandever MA, Kuehl TJ, Sulak PJ, et al. Evaluation of pituitary-ovarian axis suppression with three oral contraceptive regimens. Contraception 2008;77:162–70.
- Mishell DR, Jr. Rationale for decreasing the number of days of the hormone-free interval with use of low-dose oral contraceptive formulations. Contraception 2005;71:304–5.
- Zapata LB, Steenland MW, Brahmi D, et al. Effect of missed combined hormonal contraceptives on contraceptive effectiveness: a systematic review. Contraception 2013;87:685–700.
- Archer DF. Menstrual-cycle-related symptoms: a review of the rationale for continuous use of oral contraceptives. Contraception 2006;74:359–66.
- Kaunitz AM, Portman DJ, Hait H, et al. Adding low-dose estrogen to the hormone-free interval: impact on bleeding patterns in users of a 91-day extended regimen oral contraceptive. Contraception 2009;79:350–5.
- Miller L, Notter KM. Menstrual reduction with extended use of combination oral contraceptive pills: randomized controlled trial. Obstet Gynecol 2001;98:771–8.
- Miller L, Hughes JP. Continuous combination oral contraceptive pills to eliminate withdrawal bleeding: a randomized trial. Obstet Gynecol 2003;101:653–61.
- Edelman A, Micks E, Gallo MF, et al. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database of Syst Rev 2014;7:CD004695
- Cote I, Jacobs P, Cumming D. Work loss associated with increased menstrual loss in the United States. Obstet Gynecol 2002;100:683–7.
- Ferrero S, Abbamonte LH, Giordano M, et al. What is the desired menstrual frequency of women without menstruation-related symptoms? Contraception 2006;73:537–41.
- Sulak PJ, Cressman BE, Waldrop E, et al. Extending the duration of active oral contraceptive pills to manage hormone withdrawal symptoms. Obstet Gynecol 1997;89:179–83.
- Kwiecien M, Edelman A, Nichols MD, et al. Bleeding patterns and patient acceptability of standard or continuous dosing regimens of a low-dose oral contraceptive: a randomized trial. Contraception 2003;67:9–13.
- Dmitrovic R, Kunselman AR, Legro RS. Continuous compared with cyclic oral contraceptives for the treatment of primary dysmenorrhea: a randomized controlled trial. Obstet Gynecol 2012;119:1143–50.
- Cheewadhanaraks S, Choksuchat C, Dhanaworavibul K, et al. Postoperative depot medroxyprogesterone acetate versus continuous oral contraceptive pills in the treatment of endometriosis-associated pain: a randomized comparative trial. Gynecol Obstet Invest 2012;74:151–6.
- Vercellini P, Frontino G, De Giorgi O, et al. Continuous use of an oral contraceptive for endometriosis-associated recurrent dysmenorrhea that does not respond to a cyclic pill regimen. Fertil Steril 2003;80:560–3.
- Hall KS, Trussell J. Types of combined oral contraceptives used by US women. Contraception 2012;86:659–65.
- Andrist LC, Arias RD, Nucatola D, et al. Women’s and providers’ attitudes toward menstrual suppression with extended use of oral contraceptives. Contraception 2004;70:359–63.
- Lakehomer H, Kaplan PF, Wozniak DG, et al. Characteristics of scheduled bleeding manipulation with combined hormonal contraception in university students. Contraception 2013;88: 426–30.
- Jensen JT, Garie SG, Trummer D, et al. Bleeding profile of a flexible extended regimen of ethinylestradiol/drospirenone in US women: an open-label, three-arm, active-controlled, multicenter study. Contraception 2012;86:110–8.
- van Heusden AM, Fauser BCJM. Activity of the pituitary-ovarian axis in the pill-free interval during use of low-dose combined oral contraceptives. Contraception 1999;59:237–43.
- Reape KZ, DiLiberti CE, Hendy CH, et al. Effects on serum hormone levels of low-dose estrogen in place of placebo during the hormone-free interval of an oral contraceptive. Contraception 2008;77:34–9.
- Willis SA, Kuehl TJ, Spiekerman AM, et al. Greater inhibition of the pituitary-ovarian axis in oral contraceptive regimens with a shortened hormone-free interval. Contraception 2006;74:100–3.
- Killick SR, Fitzgerald C, Davis A. Ovarian activity in women taking an oral contraceptive containing 20 μg ethinyl estradiol and 150 μg desogestrel: effects of low estrogen doses during the hormone-free interval. Am J Obstet Gynecol 1998;179:S18–24.
- Sulak PJ, Scow RD, Preece C, et al. Hormone withdrawal symptoms in oral contraceptive users. Obstet Gynecol 2000;95:261–6.
- Schlaff WD, Lynch AM, Hughes HD, et al. Manipulation of the pill-free interval in oral contraceptive pill users: the effect on follicular suppression. Am J Obstet Gynecol 2004;190:943–51.
- Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception 2003;68:89–96.
- Anderson FD, Gibbons W, Portman D. Safety and efficacy of an extended-regimen oral contraceptive utilizing continuous low-dose ethinyl estradiol. Contraception 2006;73:229–34.
- Portman DJ, Kaunitz AM, Howard B, et al. Efficacy and safety of an ascending-dose, extended-regimen levonorgestrel/ethinyl estradiol combined oral contraceptive. Contraception 2014;89:299–306.
- Kroll R, Reape KZ, Margolis M. The efficacy and safety of a low-dose, 91-day, extended-regimen oral contraceptive with continuous ethinyl estradiol. Contraception 2010;81:41–8.
- Loseasonique (levonorgestrel/ethinyl estradiol and ethinyl estradiol) [prescribing information]. Sellersville, PA: Teva Women's Health, Inc.; 2013.
- Hickey M, Agarwal S. Unscheduled bleeding in combined oral contraceptive users: focus on extended-cycle and continuous-use regimens. J Fam Plan Reprod Health Care 2009;35:245–8.
- Darwish M, Bond M, Ricciotti N, et al. A comparison of the pharmacokinetic profile of an ascending-dose, extended-regimen combined oral contraceptive to those of other extended regimens. Reprod Sci 2014;21:1401–10.
- Darwish M, Bond M, Ricciotti N, et al. The PK/PD relationship of ethinyl estradiol and unscheduled bleeding or spotting for an ascending-dose, estrogen/progestin combination oral contraceptive (OC). Reprod Sci 2013;20(3 Suppl.):193A.
- Mendoza N, Lobo P, Lertxundi R, et al. Extended regimens of combined hormonal contraception to reduce symptoms related to withdrawal bleeding and the hormone-free interval: a systematic review of randomised and observational studies. Eur J Contracept Reprod Health Care 2014;1–19.
- Dinger J, Minh TD, Buttmann N, et al. Effectiveness of oral contraceptive pills in a large U.S. cohort comparing progestogen and regimen. Obstet Gynecol 2011;117(1):33–40.
- Howard B, Trussell J, Grubb E, et al. Comparison of pregnancy rates in users of extended and cyclic combined oral contraceptive (COC) regimens: a brief report. Contraception 2014;89:25–7.
- Archer DF, Jensen JT, Johnson JV, et al. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception 2006;74:439–45.
- Panicker S, Mann S, Shawe J, et al. Evolution of extended use of the combined oral contraceptive pill. J Fam Plan Reprod Health Care 2014;40:133–41.
- Davis MG, Reape KZ, Hait H. A look at the long-term safety of an extended-regimen OC. J Fam Pract 2010;59:E9–13
- Anderson FD, Gibbons W, Portman D. Long-term safety of an extended-cycle oral contraceptive (Seasonale): a 2-year multicenter open-label extension trial. Am J Obstet Gynecol 2006;195:92–6.
- Klipping C, Duijkers I, Fortier MP, et al. Long-term tolerability of ethinylestradiol 20 μg/drospirenone 3 mg in a flexible extended regimen: results from a randomised, controlled, multicentre study. J Fam Plan Reprod Health Care 2012;38:84–93.
- Loudon NB, Foxwell M, Potts DM, et al. Acceptability of an oral contraceptive that reduces the frequency of menstruation: the tri-cycle pill regimen. BMJ 1977;2:487–90.
- Sulak PJ, Kuehl TJ, Ortiz M, et al. Acceptance of altering the standard 21-day/7-day oral contraceptive regimen to delay menses and reduce hormone withdrawal symptoms. Am J Obstet Gynecol 2002;186:1142–9.
- Anthuber S, Schramm GA, Heskamp ML. Six-month evaluation of the benefits of the low-dose combined oral contraceptive chlormadinone acetate 2 mg/ethinylestradiol 0.03 mg in young women: results of the prospective, observational, non-interventional, multicentre TeeNIS study. Clin Drug Invest 2010; 30:211–20.
- Guilbert E, Boroditsky R, Black A, et al. Canadian Consensus Guideline on Continuous and Extended Hormonal Contraception, 2007. J Obst Gynaecol Can 2007;29:S1–32.
- Nappi RE, Pellegrinelli A, Campolo F, et al. Effects of combined hormonal contraception on health and wellbeing: women’s knowledge in northern Italy. Eur J Contracept Reprod Health Care 2015;20:36–46.
- Aubeny E, Buhler M, Colau JC, et al. Oral contraception: patterns of non-compliance. The Coraliance study. Eur J Contracept Reprod Health Care 2002;7:155–61.
- Wiegratz I, Kuhl H. Long-cycle treatment with oral contraceptives. Drugs 2004;64:2447–62.
- Stephenson J, Shawe J, Panicker S, et al. Randomized trial of the effect of tailored versus standard use of the combined oral contraceptive pill on continuation rates at 1 year. Contraception 2013;88:523–31.
- Legro RS, Pauli JG, Kunselman AR, et al. Effects of continuous versus cyclical oral contraception: a randomized controlled trial. J Clin Endocrinol Metab 2008;93:420–9.
- Hooper DJ. Attitudes, awareness, compliance and preferences among hormonal contraception users: a global, cross-sectional, self-administered, online survey. Clin Drug Invest 2010;30:749–63.
- den Tonkelaar I, Oddens BJ. Preferred frequency and characteristics of menstrual bleeding in relation to reproductive status, oral contraceptive use, and hormone replacement therapy use. Contraception 1999;59:357–62.
- Wiegratz I, Hommel HH, Zimmermann T, et al. Attitude of German women and gynecologists towards long-cycle treatment with oral contraceptives. Contraception 2004;69:37–42.
- Fruzzetti F, Paoletti AM, Lombardo M, et al. Attitudes of Italian women concerning suppression of menstruation with oral contraceptives. Eur J Contracept Reprod Health Care 2008;13:153–7.
- Merki-Feld GS, Breitschmid N, Seifert B, et al. A survey on Swiss women’s preferred menstrual/withdrawal bleeding pattern over different phases of reproductive life and with use of hormonal contraception. Eur J Contracept Reprod Health Care 2014; 19:266–75.
- Makuch MY, Duarte-Osis MJ, de Padua KS, et al. Opinion and experience of Brazilian women regarding menstrual bleeding and use of combined oral contraceptives. Int J Gynaecol Obstet 2012;117:5–9.
- Sauer U, Mann S, Brima N, et al. Offering extended use of the combined contraceptive pill: a survey of specialist family planning services. Int J Women’s Health 2013;5:613–17.
- Sanchez-Borrego R, Garcia-Calvo C. Spanish women’s attitudes towards menstruation and use of a continuous, daily use hormonal combined contraceptive regimen. Contraception 2008;77:114–17.
- Glasier AF, Smith KB, van der Spuy ZM, et al. Amenorrhea associated with contraception – an international study on acceptability. Contraception 2003;67:1–8.
- Rutter W, Knight C, Vizzard J, et al. Women’s attitudes to withdrawal bleeding and their knowledge and beliefs about the oral contraceptive pill. Med J Aust 1988;149:417–19.
- A cross-cultural study of menstruation: implications for contraceptive development and use. World Health Organization Task Force on Psychosocial Research in Family Planning, Special Programme of Research, Development and Research, Training in Human Reproduction. Stud Fam Planning 1981;12:3–16.
- Makuch MY, Osis MJ D, de Padua KS, et al. Use of hormonal contraceptives to control menstrual bleeding: attitudes and practice of Brazilian gynecologists. Int J Women’s Health 2013;5:795–801.
- Archer DF, Kovalevsky G, Ballagh SA, et al. Ovarian activity and safety of a novel levonorgestrel/ethinyl estradiol continuous oral contraceptive regimen. Contraception 2009;80:245–53.
- Kroll R, Seidman L, Ricciotti N, et al. A phase 1, multicentre, open-label study to evaluate ovarian follicular activity and hormone levels with an extended-regimen combined oral contraceptive with low-dose ethinyl estradiol supplementation. Eur J Contracept Reprod Health Care 2015;20:249–58.
- Davis AR, Kroll R, Soltes B, et al. Occurrence of menses or pregnancy after cessation of a continuous oral contraceptive. Fertil Steril 2008;89:1059–63.
- Anderson FD, Hait H, Hsiu J, et al. Endometrial microstructure after long-term use of a 91-day extended-cycle oral contraceptive regimen. Contraception 2005;71:55–9.
- Anderson FD, Feldman R, Reape KZ. Endometrial effects of a 91-day extended-regimen oral contraceptive with low-dose estrogen in place of placebo. Contraception 2008;77:91–6.
- Seval DL, Buckley T, Kuehl TJ, et al. Attitudes and prescribing patterns of extended-cycle oral contraceptives. Contraception 2011;84:71–5.
- Sulak PJ, Buckley T, Kuehl TJ. Attitudes and prescribing preferences of health care professionals in the United States regarding use of extended-cycle oral contraceptives. Contraception 2006;73:41–5.
- Frederick CE, Edelman A, Carlson NE, et al. Extended-use oral contraceptives and medically induced amenorrhea: attitudes, knowledge and prescribing habits of physicians. Contraception 2011;84:384–9.
- Gerschultz KL, Sucato GS, Hennon TR, et al. Extended cycling of combined hormonal contraceptives in adolescents: physician views and prescribing practices. J Adolesc Health 2007;40:151–7.
- Wiegratz I, Galilaer K, Sanger N, et al. Prescribing preferences and personal experience of female gynaecologists in Germany and Austria regarding use of extended-cycle oral contraceptives. Eur J Contracept Reprod Health Care 2010;15:405–12.
- Pompei LM, Fernandes CE, Steiner ML, et al. Attitudes, knowledge and prescribing habits of Brazilian gynecologists regarding extended-cycle oral contraceptives. Gynecol Endocrinol 2013;29: 1071–4.
- Female OB/GYNs speak out about health practices. AWHONN Lifelines 2004;8:14–8.
- Frank E, Elon L. Clinical and personal relationships between oral contraceptive and hormone replacement therapy use among US women physicians. Menopause 2003;10:133–41.
- Dehlendorf C, Krajewski C, Borrero S. Contraceptive counseling: best practices to ensure quality communication and enable effective contraceptive use. Clin Obstet Gynecol 2014;57:659–73.
- Nobili MP, Piergrossi S, Brusati V, et al. The effect of patient-centered contraceptive counseling in women who undergo a voluntary termination of pregnancy. Patient Educ Couns 2007;65:361–8.
- Gemzell-Danielsson K, Thunell L, Lindeberg M, et al. Comprehensive counseling about combined hormonal contraceptives changes the choice of contraceptive methods: results of the CHOICE program in Sweden. Acta Obstet Gynecol Scand 2011;90:869–77.
- Merki-Feld GS, Gruber IM. Broad counseling for adolescents about combined hormonal contraceptive methods: the choice study. J Adolesc Health 2014;54:404–9.
- Halpern V, Lopez LM, Grimes DA, et al. Strategies to improve adherence and acceptability of hormonal methods of contraception. Cochrane Database Syst Rev 2011;(4):CD004317
- Wiegratz I, Stahlberg S, Manthey T, et al. Effects of conventional or extended-cycle regimen of an oral contraceptive containing 30 mcg ethinylestradiol and 2 mg dienogest on various hemostasis parameters. Contraception 2008;78:384–91.
- Wiegratz I, Stahlberg S, Manthey T, et al. Effects of an oral contraceptive containing 30 mcg ethinyl estradiol and 2 mg dienogest on lipid metabolism during 1 year of conventional or extended-cycle use. Contraception 2010;81:57–61.
- Machado RB, de Melo NR, Maia H, Jr., et al. Effect of a continuous regimen of contraceptive combination of ethinylestradiol and drospirenone on lipid, carbohydrate and coagulation profiles. Contraception 2010;81:102–6.
- Hillard PA. Menstrual suppression: current perspectives. Int J Women’s Health 2014;6:631–7.
- Jacobson JC, Likis FE, Murphy PA. Extended and continuous combined contraceptive regimens for menstrual suppression. J Midwifery Women's Health 2012;57:585–92.
- Nelson AL. Extended-cycle oral contraception: a new option for routine use. Treat Endocrinol 2005;4:139–45.
- Nelson AL. Communicating with patients about extended-cycle and continuous use of oral contraceptives. J Women’s Health 2007;16:463–70.
- Ong LML, de Haes JCJM, Hoos AM, et al. Doctor-patient communication: a review of the literature. Soc Sci Med 1995;40:903–18.