Abstract
Purpose. Supplemental administration of androgens has been advocated for men with sexual dysfunction (SD) and hypoandrogenism. The preponderance of evidence indicates that most delivery forms of testosterone (T) are effective but the role of dehydroepiandrosterone (DHEA) is controversial. A placebo-controlled, randomized trial of oral androgen (T versus DHEA) supplementation was carried out to determine their efficacy.
Materials and methods. Eighty-six men with SD and decreased levels of serum T and/or DHEA, participated in a study receiving oral T undecanoate (OTU) (n = 29) 80 mg twice daily, DHEA (n = 28) 50 mg twice daily, or placebo (n = 29). Outcomes included evaluation of sexual performance by the International Index of Erectile Function (IIEF), the Androgen Deficiency in the Aging Male (ADAM), Aging Male Symtom Scale (AMS), and Global Assessment Questionnaire (GAQ) questionnaires. Biochemical evaluations included measurement of T and DHEA, prolactin, gonadotropins, and PSA.
Results. Seventy-nine men completed the study. There were no significant differences in outcomes as assessed by four different instruments: the ADAM, IIEF, AMS, and GAQ in regard to sexual interest or erectile function. Biochemically, a significant increase in serum DHEA between baseline and final visit was documented in the group receiving DHEA. The levels of T, on the other hand, increased insignificantly between entry and final visit in the T cohort. No biochemical changes were observed in the placebo group. Levels of PSA remained stable in all three groups.
Conclusions. This study did not suggest a clinical benefit of OTU or DHEA supplementation in men with hypoandrogenism and SD. The recommended dose of OTU may have been inadequate or poorly absorbed. Increased doses or an alternative T delivery form may result in a different response.
Introduction
An adequate androgen milieu is fundamental for satisfactory sexual function. In animals, hypogonadism results in marked deterioration in libido and severe alterations in the smooth muscle of the corpora cavernosa leading to erectile failure which can be corrected by testosterone (T) treatment [Citation1].
A decline in androgen production is frequently associated with advancing age but there is a great inter-individual variability in the development of the T deficiency syndrome (TDS) [Citation2]. The decline in the serum levels of dehydroepiandrosterone (DHEA) and its sulfate (DHEAS), in association with aging, is a more common occurrence. It also develops earlier than TDS and in a more predictable manner but its significance, as a potential health issue, remains to be determined. Administration of T and DHEA has been advocated for men with sexual dysfunction (SD) and documented T or DHEA deficiency. Previous studies have consistently supported the efficacy of oral [Citation3,Citation4], transdermal [Citation5], and injectable T [Citation6]. Controversy on the efficacy of T centers, primarily, around the specific formulation of the oral undecanoate has been reported recently as ineffective [Citation7,Citation8]. Regarding DHEA, early studies on its usefulness in treating SD were promising [Citation9] but, again more recent evidence has questioned the validity of those findings when treatment relied exclusively on DHEA supplementation [Citation10]. Despite the controversy, both T and DHEA are considered central neurosteroids [Citation11] and have been found to have direct effects on endothelial nitric oxide synthase activation and induction which are fundamental events in the mechanisms involving libido and penile erections [Citation12,Citation13].
To better define the role of DHEA in sexual performance and based on the concepts that DHEA is convertible into T [Citation14] and that administration of DHEA results in biosynthesis of active androgens by tissue targets without representation in the peripheral circulation [Citation15], we explored the effect of either T or DHEA administration to men with SD and hypoandrogenism in a controlled study.
Materials and methods
Patients and inclusion criteria
Participants were men 45–70 years of age recruited from ambulatory clinics and with a primary complaint of SD. The main inclusion criteria were a clinical picture compatible with TDS; a history of SD for >3 months with International Index of Erectile Function (IIEF) scores of <25 on questions 1–5 and 15; a history of hypoactive sexual desire disorder (HSDD) for >3 months with scores of >6 for questions 11 and 12 of the IIEF and serum total T levels <12 nmol/l and DHEAS of <3.5 μmol/l. Men were excluded if there was a contraindication for the use of androgens, including a history of prostate cancer, Prostate Specific Antigen (PSA) levels >4 ng/l, or a history of substance abuse within 2 years. If a patient had received T supplementation, it had to be discontinued for >6 weeks in the case of injectable preparations (enanthate or cypionate) or >2 weeks for oral or transdermal delivery formulations. Before entry, patients received a thorough explanation of the experimental nature of the study. The protocol received institutional ethics approval for each individual site.
Clinical trial registry
This trial was registered at www.clinicaltrials.gov under number NCT00202163.
Instruments and procedures
Baseline and primary clinical outcomes included evaluation of sexual performance and interest by self-reported instruments including: (a) the IIEF [Citation16], a validated questionnaire with specific domains for erectile and libido assessment; (b) the Androgen Deficiency in Aging Men (ADAM) questionnaire, specifically designed for screening of hypogonadism [Citation17]; (c) the Aging Male Symptom Scale (AMS) [Citation18], a symptom rating scale expressly developed as an outcome measure for androgen treatment which consists of three separate categories: two symptom domains (psychological and somatovegative) and sexual complaints section; and (d) Global Assessment Questionnaire (GAQ) questionnaire which asks the patients about their subjective assessment of benefits from the treatment. Biochemical evaluations included measurement of serum T and DHEA, prolactin, gonadotropins, and PSA on two separate blood samples taken between 07:00 and 11:00 h, at entry and within 6 h of the last dose. The pre-designated primary outcomes were the erectile and desire domains of the IIEF.
Men fulfilling the criteria were offered participation in a placebo-controlled, randomized, double blind, double dummy, fixed dose study of 4 months duration, receiving either oral T undecanoate (OTU) (Androgel Testocaps, Organon/Schering-Plough, Oss, The Netherlands) 80 mg twice daily, DHEA 50 mg twice daily (specifically manufactured and tested for solubility and bioavailability for this trial by Paladin Labs. Montreal, Canada) or placebo (provided by the manufacturers). Patients were carefully advised to take the medications with meals since it is a fundamental requirement for OTU absorption. Participants were allocated at random to one of the three cohorts. Blinded treatment packages were prepared and assigned by a central pharmacist using randomly permuted blocks of size nine.
Statistical analysis
Changes in the biochemical values from baseline to follow-up were compared between arms by the Kruskal–Wallis test. Significant within arm biochemical changes were identified by the Wilcoxon signed rank test. The change in the IIEF total and domain scores and the AMS total score were compared between the two active treatment arms and the placebo arm by analysis of covariance adjusting for the baseline score. Dunnett's method was used for the comparison of the two active treatment and the placebo groups. In accordance to our pre-specified analysis plan, we did not compare the two active treatment arms unless at least one of the arms improved significantly more than placebo.
Conditional logistic regression, stratified by patient, was used to test if the pre- versus post odds ratio of reporting decreased libido or erection strength differed between groups. The ordinal follow-up libido and function global assessment scores were compared between groups by the Kruskal–Wallis test. All tests were two-sided and analysis was performed in SAS V9.1 (SAS Institute SAS/STAT9.1-User's Guide. Cary NC: SAS Publishing, 2004).
Results
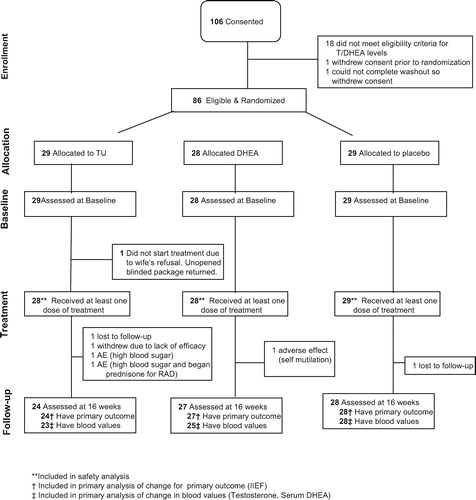
One hundred six men from four centers participated. Of these, 21 dropped out before receiving medication (). Safety evaluation includes the 85 patients who received at least one dose of medication while the primary efficacy analysis is based on the 79 patients who completed the 16-week assessment. Baseline characteristics are presented in .
Table I. Baseline patient characteristics of efficacy analysis dataset.
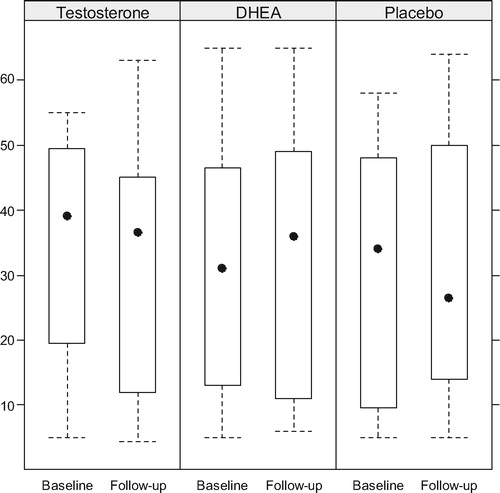
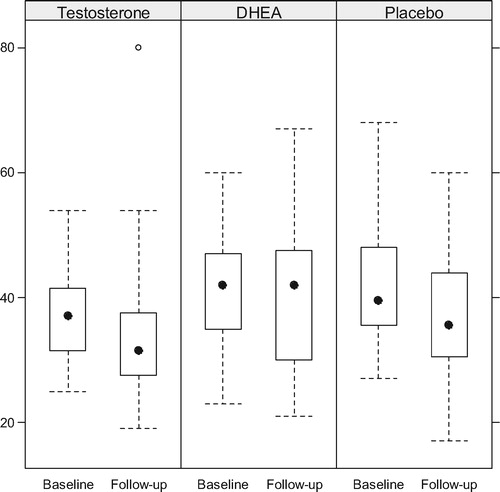
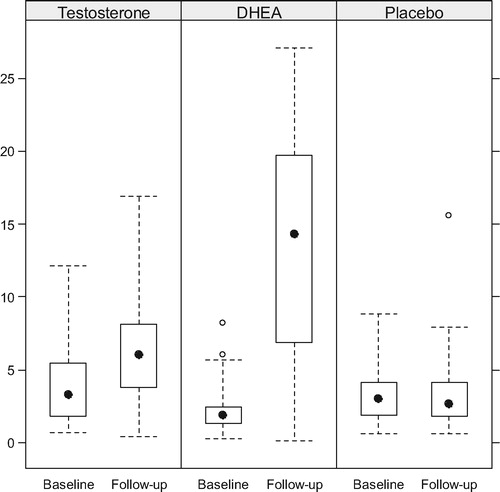
Boxplots of the T and serum DHEA levels at baseline and follow-up are presented by arm in and , while compares the within patient changes in biochemical levels between treatment arms. Serum T increased significantly in the DHEA arm (p = 0.016) but not in the OTU or placebo arms. Serum DHEA increased significantly in both the DHEA and TU arms (both p < 0.001), although the increase was much larger in the DHEA arm than the OTU arm (p < 0.001). Gonadotropins decreased significantly only in the OTU and DHEA in the arms. Prolactin and PSA remained stable in all arms.
Table II. Change in biochemical values.
Figure 2. Serum T levels (nmol/L) before and upon completion of treatment with TU. Although T levels increased the intra- or inter-group differences were not significant.
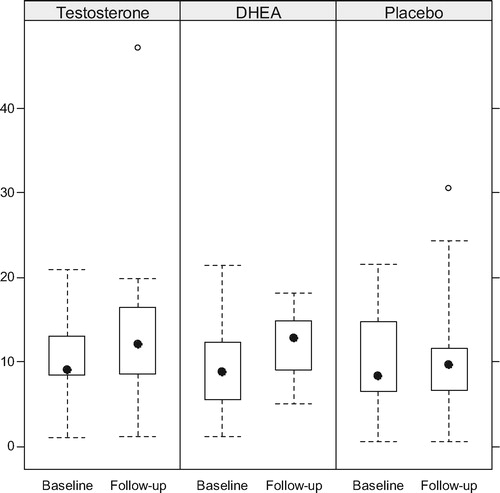
Figure 3. Changes in the levels of serum DHEA. There was a modest increase in the T group and a highly significant increase in the DHEA group.
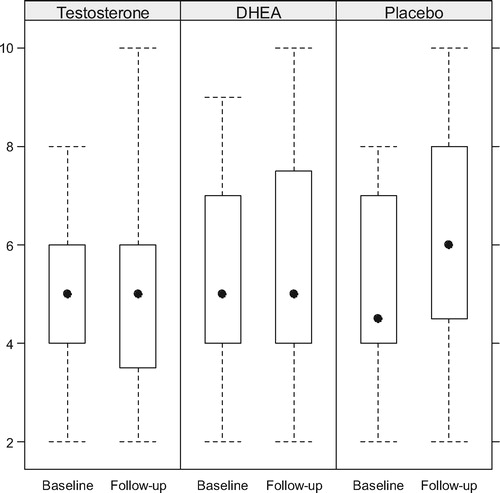
Baseline and follow-up IIEF and AMS values appear in . Little change occurred in IIEF domains or AMS scores in any arm; there was no suggestion that the OTU or DHEA cohorts experienced a clinical response different than placebo ().
Table III. Comparison of change in IEEF and AMS*.
Figure 5. Comparative scores in the desire domain of the IIEF. The differences were barely significant only in the placebo group.
Between baseline and follow-up, the overall number reporting poor libido (ADAM question 1) and erection strength (ADAM question 7) decreased from 68 (86%) to 49 (62%) (p < 0.001) and from 74 (94%) to 63 (80%), respectively (p = 0.003). This improvement was uniform across all arms ().
Table IV. Adam questionnaire.
Distribution of libido and function global assessment scores were similar across treatments, with some improvement reported in 22 (92%), 26 (96%), and 24 (86%) of patients in the TU, DHEA, and placebo arms, respectively for both scores (). As a sensitivity analysis we used the dichotomized GAQ scores assuming that all randomized patients who dropped out in the placebo arm (n = 1) improved while all those who dropped out in the treatment arms (n = 6) did not improve, then repeated the analysis under the reverse assumptions. The proportion of patients with improved libido and/or function was not significantly different between arms under either of these extreme missing data assumptions.
Table V. GAQ at follow-up.
In keeping with their main complaint of poor libido and erectile capacity, of the 79 evaluable patients, only 49 (62%) had made at least one attempt at sexual intercourse during the 4 weeks prior to study enrollment and 47 (59%) made at least one attempt during the 4 weeks before the final follow-up visit. Clinical outcomes were re-examined in the subgroup of 39 (49%) patients who attempted sexual intercourse in the 4 weeks prior to baseline and follow-up. Although excluding patients who did not attempt sexual intercourse at both time points uniformly increased the IIEF scores, there remained no suggestion that IIEF improved more in either of the active treatment arms than in the placebo arm. All the other outcomes remained substantially unchanged from the primary analysis (data not shown).
Adverse effects were uncommon. In the OTU group hyperglycemia and dyspepsia occurred in 2 (7%) and 1(4%), respectively; these were attributed to the medication and are recognized adverse effects of this preparation. One man in this group developed a melanoma, not considered related to OTU. In the DHEA group one man developed psychiatric alterations with an attempt at self-mutilation; this was considered as probably drug-related. There were no adverse effects in the placebo group.
Discussion
There is reliable evidence in the literature supporting the concept that T supplementation is beneficial to men with SD when used alone [Citation3,Citation5,Citation6,Citation19], or in combination with other erectogenic drugs [Citation4,Citation19]. Recently, its usefulness has been challenged [Citation7]. The controversy frequently relates to several factors including inadequate selection of patients, such as those who are not truly hypogonadal, the duration of the study or the choice of outcome parameters employed for assessment of efficacy. However, the fundamental discrepancies relate to the formulation studied. In the case of OTU recent results clearly show that its effectiveness in SD is questionable. Emmelot-Vonk et al. [Citation7] reported that OTU is unsuccessful, not only in treating SD but, in a separate report on the same population, it failed to improve cognitive function or bone mineral density, the latter not surprisingly, since the trial was of only 6 months duration [Citation20]. The study of Legros et al. [Citation8] also using OTU and our current study, both focusing exclusively on sexual function, further confirm the lack of efficacy of this particular T delivery formulation. Although the clinical responses were disappointing in the three studies, there were some significant differences in design and execution of the trials which are worth noting. In the Emmelot-Vonk et al. study only a small proportion had ED (about 11%) or lack of sexual interest (about 12%) while between 20 and 30% had not attempted sexual activity for >4 weeks. In addition, the whole group was borderline hypogonadal (serum T 10–11 nmol/l) and, the single clinical outcome measure was the ‘Eleven Questions on Sexual Functioning Questionnaire’; the number of participants was large (n = 207). The investigation by Legros et al. [Citation8] also included a large number of subjects (n = 322), but with a significant number of them not fulfilling the recommended criteria for T deficiency [Citation21] (mean total serum T level 14.9 ± 5.52 nmol/l) in a single sampling and with some subjects ranking in the upper eugonadal range (>30 nmol/l); they were allowed to participate because of a criterion based on conversion of the results to a calculated free T. As in our protocol, they employed the ADAM questionnaire for initial screening. In addition to placebo, participants were distributed into three additional arms in which participants received fixed daily doses of OTU (80, 160, or 240 mg, respectively). The study outcomes were based on the AMS rating scale and the Derogatis sexual functioning self-report. The article, however, is silent as to any changes in the serum T levels during or upon completion of the treatment period. In our study the definition of hypoandrogenism was stricter and closer to recent recommendations [Citation20], blood samples were obtained at screening, baseline and last visit for assessment of androgens levels and 4 questionnaires were employed as outcome measures. A limitation of our study, on the other hand, is the smaller number of participants, as compared to the other two studies.
The IIEF is widely recognized as a valid instrument for assessment of erectile function, libido and various aspects of sexual performance. Our analysis of the whole questionnaire or the specific domains of erectile function and desire failed to show any changes that would have occurred as a result of administration of either oral T or DHEA.
The AMS is a fully validated and frequently used tool that assesses not only sexual function but various other manifestations including sense of well being, sleep disturbances, mood, etc. that are commonly associated with TDS. Our finding of a lack of significant changes in the active arms compared to placebo in any of its parameters following 16 weeks of treatment with an oral androgen provides strong supportive confirmation of the results recorded independently, by Legros et al. [Citation8] with the same instrument.
The ADAM is a simpler instrument, useful for screening of TDS that includes 1 question each on erectile function and sexual desire and 8 other inquiries on possible manifestations of hypogonadism. The improvement noted from baseline to follow-up was similar in all arms including placebo, thus, confirming a lack of effect with the use of oral T or DHEA.
A different oral T preparation (methyl T) was reported to be effective in improving sexual performance [Citation2]. Methylated T oral delivery forms exhibit significant liver toxicity and have rightly, been removed from the market. Currently TU is the only oral formulation available that is free of liver toxicity but it is recognized to have absorption issues that can be potentially circumvented by ingesting it simultaneously with a fatty meal. The manufacturer only indicates that it ‘must be taken with a meal’. However, evaluation of the OTU preparation used in this study, has shown that a low-calorie (<220 kcl), low-lipid (<5 g) meal results in inadequate absorption of the drug that can be avoided when the lipid intake per meal, reaches more than 19 g [Citation22]. This requirement may present an inconvenience to many men, particularly for the morning dose. Compliance with taking the medication, similarly to other studies [Citation7,Citation8] was not a problem and was readily corroborated by the number of pills/capsules returned; compliance with the dietary recommendations was exclusively based on participants' reports and not verifiable. None of the participants used cholesterol absorption inhibitors such as ezetimibe.
The daily DHEA dose (100 mg) was selected because of the results of a shorter (3 months), open label trial reported by Flynn et al. [Citation23]. We documented excellent serum levels, confirming the satisfactory absorption of this particular preparation of the hormone. The increase in T levels in this group would add to the concept of conversion of DHEA into T, previously documented in the study of Flynn et al. [Citation23]. The absence of a sexual response in the presence of a good biochemical one indicates that DHEA alone has no role to play in treating either erectile dysfunction or male HSDD. Although a positive effect of DHEA in sexual domains has been reported [Citation9], the preponderance in the literature [Citation10,Citation23,Citation24] and our study indicate that the role of DHEA is insignificant. Our findings also speak against the concept of DHEA as a neurosteroid with a central putative effect on libido [Citation25] although the hormone has been found to have an effect on self-esteem and mood in younger individuals with Addison's disease [Citation26]. While DHEA has been documented to have direct genomic and non-genomic effects on the vascular endothelium [Citation12,Citation13], its clinical effect as a modulator of endothelial function with a role as a facilitator of penile erections was not evident from our results.
The 16-week duration chosen for the trial was based on the fact that all studies assessing the efficacy of T administration to hypogonadal men have shown that the sexual response is defined within the first 12 weeks of treatment [Citation3,Citation6,Citation27]. Although it is possible that a larger dose might produce better results, Legros et al. [Citation8] reported equally negative findings with oral doses of TU as high as 240 mg daily. A possible explanation is that the patients did not carefully adhere to the dietary instructions about concomitant fatty food ingestion necessary for an appropriate absorption of the hormone and that the recommendation of simply taking it with meals (as done in other studies) is not sufficiently adequate. The administration of DHEA, on the other hand, resulted in a marked increase in serum levels not only of DHEA but also a modest but significant increase in serum T, confirming that the formulation of DHEA used in this study was well absorbed and resulted in adequate serum levels. The increase in the levels of T in the cohort receiving DHEA, suggests that some conversion of DHEA to T does take place (), while the opposite does not.
To our knowledge, a comparative trial of any T formulation versus DHEA in men with SD is not available in the literature and our study adds to the understanding of the role of androgens in sexual function. The absence of response, particularly in the OTU group might be related to the dose used. A flexible-dose design, allowing the patients to titrate up the dose according to the response, might have obviated this problem. In our situation, with a three arm design, the need for a large increase in the number of placebo and dummy pills/capsules would have created insurmountable difficulties for the execution of the trial. Nevertheless, considering the results of a study with larger doses of OTU [Citation8], it is doubtful that such approach would have improved the results.
Conclusions
Although the results of the trial are negative, its external validity is of clinical relevance and provides confirmatory support of recent studies investigating OTU. In addition, DHEA does not have a significant effect in men with deficiency of T or DHEA and SD, even if physiological serum levels are obtained with DHEA supplementation. Since T administration by the intramuscular [Citation6], transdermal [Citation5,Citation19] and oral [Citation4] routes, including OTU [Citation4], has been found to improve libido and erectile function with or without the concomitant use of a phosphodiasterase-5 inhibitor, we believe that the negative results reported herein are, primarily, a problem of the chosen formulation (OTU) rather than an issue of T efficacy in sexual performance.
Acknowledgements
We are grateful to the sponsor for providing the study medication, including placebos as well as financial support for laboratory testing and data collection. The study was conceived, designed and executed by the authors without advice, supervision or oversight of quality control by the sponsors. The statistical analysis and the writing of this report were also done exclusively by the authors without participation of the sponsors. The study received financial support from Organon Canada and Paladin Laboratories who also provided the drugs used. The Canadian Society for the Study of the Aging Male made available initiation support. Dr. Richard Casey, Oakville, Ont. also participated. Andrey Pavlov assisted with the statistical analysis.
References
- Traish AM, Guay AT. Are androgens critical for penile erections in humans? Examining the clinical and preclinical evidence. J Sex Med 2006;3:382–407.
- Diver MJ. Analytical and physiological factors affecting the interpretation of serum testosterone concentrations in man. Ann Clin Biochem 2006;43:3–12.
- Morales A, Johnston B, Heaton JPW, Lundie M. Testosterone supplementation in hypogonadal impotence: assessment of biochemical measures and therapeutic outcomes. J Urol 1997;157:849–852.
- Kalinchenko SY, Kozlov GI, Gontcharov NP, Katsiya GV. Oral testosterone undecanoate reverses erectile dysfunction associated with diabetes mellitus in patients failing on sildenafil citrate therapy alone. Aging Male 2003;6:94–97.
- Wang C, Swerdloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G, et al Transdermal testosterone gel improves sexual function, mood muscle strength and body composition parameters in hypogonadal men. Testosterone gel study group. J Clin Endocrinol Metab 2000;85:2839–2853.
- Jockenhövel F, Minnemann T, Schubert M, Freude S, HüblerD, Schuman C, Christoph A, Ernst M. Comparison of log-acting testosterone undecanoate formulation versus testosterone enanthate on sexual function and mood in hypogonadal men Eur J Endocrinol 2009;160:815–819.
- Emmelot-Vonk MH, Verhaar HJJ, Nakhai-Pour, Grobee DE, van der Schouw YT. Effect of testosterone supplementation on sexual functioning in aging men: a randomized controlled trial. Int J Impotence Res 2009;21:129–138.
- Legros JJ, Meuleman EJ, Elbers JM, Geurts TB, Kasper MJ, Bouloux PM. Oral testosterone replacement in symptomatic late-onset hypogonadism: effect on rating scales and general safety in a randomized, placebo controlled study. Eur J Endocrinol 2009;160: 821–831.
- Reiter WJ, Pycha A, Schatzel G, Pokorny A, Gruber DM, Huber J, Maberger M. Dehydroepiandrosterone in the treatment of erectile dysfunction: a prospective, double-blind, randomized, placebo-controlled study. Urology 1999;53:590–595.
- Nair KS, Rizza AR, O'Brien P, Dhatariya K, Short KR, Nhera A, Vitone JL, Klee GG, Basu N, Basu A, et al DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 2006;355:1647–1659.
- Saad F, Hoesl CE, Oettel M, Fauteck JD, Römmeler A. Dehydroepiandrosterone treatment in the aging male – what should the urologist know. Eur Urol 2005;48:724–733.
- Bernini G, Versari D, Moretti A, Virdis A, Ghiadoni L, Bardini M, Taurino C, Canale D, Saveti A. Vascular reactivity in congenital hypogonadal men before and after testosterone replacement therapy. J Clin Endocrinol Metab 2006;91:1691–1696.
- Williams MRI, Dawood T, Ling S, Dai A, Lew A, Kraus L. Dehydroepiandrosterone increases endothelial cell proliferation in vitro and improves endothelial function in vivo by mechanisms independent of androgen and estrogen receptors. J Clin Endocrinol Metab 2004;89:4708–4715.
- Baulieu EE, Thomas G, Legrain S, Lahlou N, Roger M, Dabuire B, Faucounau V, Girard L, Harvy MP, Latour F, et al Dehydroepiandrosterone (DHEA), DHEA sulfate and aging: contribution of the DHEAge Study to a sociobiomedical issue. PNAS 2000;97:4279–4290.
- Labrie F, Belanger A, Cusan L, Candas B. Physiological changes in dehidroepiandrosterone are not reflected by serum levels of active androgens and estrogens but of their metabolites: Intracrinology. J Clin Endocrinol Metab 1997;82:2403–2409.
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, et al The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822–830.
- Morley JE, Perry HM II, Kevorkian RT, Patrick P. Comparison of screening questionnaires for the diagnosis of hypogonadism. Maturitas 2006;53:424–429.
- Kratzik C, Heinemann LA, Saad F, Thai DM, Rücklinger E. Composite screener for androgen deficiency related to the Aging Males' Symptoms scale. Aging Male 2005;8:157–161.
- Shabsigh R, Kaufman JL, Steidle C. Padma-Nathan H. Randomized study of testosterone gel as adjunctive therapy to sildenafil in hypogonadal men with ED who do not respond to sildenafil alone. J Urol 2004;172:658–663.
- Emmelot-Vonk, Verhaar HJJ, Pour HRN, Aleman A, Lock TMTW, Bosch JL, Grobee DE, van der Schouw YT. Effect of testosterone supplementation on functional mobility, cognition and other parameters in older men. JAMA 2008;299:39–52.
- Wang C, Nieschlag E, Swerdloff R, Behre H, Hellstrom WY, et al Investigation, treatment and monitoring of late onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Aging Male 2009;12:5–12.
- Schnabel PG, Bagchus W, Lass H, Thomsen T, Geurts TB. The effect of food composition on serum testosterone levels after oral administration of Andriol Testocaps. Clin Endocrinol (Oxf) 2007;66:579–584.
- Flynn MA, Weaver-Osterholtz D, Sharpe-Timms KL, AllenS, Krause G. Dehydroepiandrosterone replacement in aging humans. J Clin Endocrinol Metab 1999;84:1527–1533.
- Løvas K, Gebre-Medhin G, Trovik TS, Fougner KJ, UhlvingS, et al Replacement of dehidroepiandrosterone in adrenal failure: No benefit for subjective health status and sexuality in a 9-month, randomized, parallel group clinical trial. J Clin Endocrinol Metab 2003;88:1112–1118.
- Hunt PJ, Gurnell EM, Huppert FA, Richards C, Prevost AT, Waas JA, Herbert J, Chaterjee VK, Improvement in mood and fatigue after dehidroepiandrosterone replacement in Addison's disease in a randomized, double blind trial. J Clin Endocrinol Metab 2000;85:4650–4655.
- Acherman JC, Silverman BL. Commentary: dehydroepiandrosterone replacement for patients with adrenal insufficiency. Lancet 2001;357:1381.
- Saad F, Gooren LJ, Haider A, Yassin A. A dose-response study of testosterone on sexual dysfunction and features of the metabolic syndrome using testosterone gel and parenteral testosterone undecanoate. J Androl 2008;29:102–107.