Abstract
Objective. In the few studies to evaluate antimuscarinics for overactive bladder (OAB) in men, safety has been the primary focus. In OAB efficacy trials, subject populations have been predominantly female and patient-reported outcomes (PROs) have been assessed only recently. We present a post hoc analysis of PRO-based findings from the subset of men (without presumed bladder outlet obstruction [BOO]) from two large, independent, open-label trials of solifenacin.
Methods. Subjects with OAB for ≥3 months received flexibly dosed solifenacin for 12 weeks. At baseline and 4-week intervals, subjects completed the Patient Perception of Bladder Condition (PPBC) and Overactive Bladder Questionnaire (OAB-q). In one study, subjects also completed 3-day bladder diaries. At baseline, mean PPBC scores were similar in both studies and indicative of moderate-to-severe problems.
Results. After 12 weeks of solifenacin, mean PPBC scores improved significantly (p < 0.0001); values were suggestive of minor-to-moderate problems. Mean scores on the OAB-q were also significantly improved after solifenacin (p values ≤0.001). In men without presumed BOO, solifenacin significantly improved PRO measures of symptom bother, health-related quality of life, and overall perception of bladder problems.
Conclusion. Results from these two studies support the use of solifenacin as a well-tolerated and efficacious treatment option for providing symptom relief in men with OAB without BOO.
Introduction
In 2002, the International Continence Society proposed the term, ‘lower urinary tract symptoms (LUTS)’ to describe the spectrum of complaints associated with the storage and voiding phases of micturition in an effort to mitigate confusion regarding the etiology of these symptoms and, importantly, to avert misdiagnosis and inappropriate management [Citation1]. Storage symptoms include urgency, urinary incontinence (UI), frequency, and nocturia; voiding symptoms include hesitancy, slow stream, terminal dribble, and the feeling of incomplete emptying. A subset of storage symptoms – urgency, with or without urgency UI, often with increased daytime frequency and nocturia – define the overactive bladder (OAB) syndrome [Citation1,Citation2].
Although the term ‘LUTS’ is accepted and used in research and writing, clinical dogma regarding etiology and treatment remains essentially unchanged. In men, LUTS are generally attributed to prostatic pathology, including benign prostatic hyperplasia (BPH) and bladder outlet obstruction (BOO), and first-line treatment typically involves an α1-receptor antagonist and/or 5α-reductase inhibitor [Citation3]. However, it is becoming increasingly clear that not all male LUTS are due to prostate problems. The bladder is often involved, as are other extra-urogenital factors. Survey data from a large, population-based study showed that the prevalence of self-reported LUTS in men was 63%, and rates increased from 51% in men aged ≤39 to 81% in those ≥60 years. Storage symptoms were twice as common as voiding symptoms (51% vs. 26%), and OAB was reported by 11% of male respondents [Citation4]. Thus, it is likely that male patients presenting with LUTS will have highly variable constellations of problems characterized by both storage and voiding symptoms. The bottom line: not all male LUTS, particularly storage LUTS, are due to BPH/BOO.
Owing to the development of effective pharmacotherapies for male LUTS, with or without BPH/BOO, there has been a shift away from surgery to medication management. In turn, a larger number of patients are presenting to their primary care physician (PCP) with bothersome symptoms [Citation5]. Thus, it is imperative that PCPs should properly evaluate the patients with tools readily available to them and prescribe medications likely to optimize the treatment responses. To date, research has shown that PCPs are more likely than are urologists to use watchful waiting versus medication. When PCPs do prescribe, they more often write for nonselective α-blockers. By contrast, urologists more often write for 5-α-reductase inhibitors (5-ARIs), a 5-ARI plus an α-blocker, or an antimuscarinic [Citation5].
Antimuscarinics can often reduce OAB symptoms [Citation6] and improve patient-reported outcomes (PROs) [Citation7] in men and women. Although antimuscarinics are prescribed as first-line therapy for OAB symptoms in women, the therapeutic imperative for their use in men is less clear owing to the potential for multiple etiologies. Reluctance to use antimuscarinics may also reflect concerns that these agents may cause acute urinary retention (AUR) in men with possible or presumed BOO. However, placebo-controlled trials have shown that AUR is rare in men not presenting with large post-void residual (PVR) volumes [Citation8]. Moreover, evidence that antimuscarinics can be used safely in carefully selected men with OAB symptoms with or without BOO continues to accumulate (see reviews by Chapple and Roehrborn [Citation3] and Kaplan [Citation9]).
Owing to the paucity of published PRO data on antimuscarinics in men, post hoc analyses of PRO data were undertaken on ∼450 presumed unobstructed men from two open-label clinical trials of solifenacin: the VESIcare Open-Label Trial (VOLT) [Citation10] and the VESIcare Efficacy and Research Study US (VERSUS) [Citation11]. We also report results for diary-based endpoints in male subjects from VERSUS given that few studies have been conducted in this subpopulation with OAB. Our intent was to explore the impact of OAB symptoms and the effects of treatment on these symptoms in an under-studied subgroup of patients with OAB.
Methods
VOLT and VERSUS were open-label, multicenter, phase IIIb clinical trials, and details regarding the methods and results for the full study populations are published [Citation10,Citation11]. Briefly, each 12-week study allowed for flexible dosing of solifenacin whereby subjects started at an oral dose of 5 mg/day with dose adjustment to 10 mg possible at Weeks 4 or 8 (VOLT only) if well-tolerated ().
Figure 1. Summarized timelines comparing VOLT and VERSUS. VOLT, VESIcare Open-Label Trial; VERSUS, VESIcare Efficacy and Research Study US; PPBC, Patient Perception of Bladder Condition; OAB-q, Overactive Bladder Questionnaire; OAB, overactive bladder.
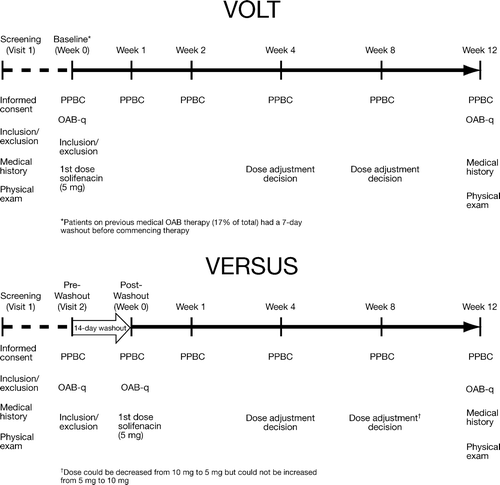
VOLT enrolled 2,225 treatment-naïve and experienced subjects (395 men), while VERSUS enrolled 441 subjects (52 men) who switched from tolterodine extended release (ER) to solifenacin owing to residual urgency symptoms. Eligible subjects had OAB symptoms for ≥3 months. VOLT was designed to assess treatment-related changes in PROs only; VERSUS was designed to assess treatment-related changes in both diary-based and PROs. For VERSUS, subjects must have received tolterodine ER for ≥4 weeks without sufficient improvement in urgency episodes (mean of ≥3 urgency episodes/24 h). Baseline values were recorded before and after cessation of tolterodine ER (2-week washout period); Post-Washout values were used here for comparison with VOLT. Men were excluded if they had clinically significant BOO as determined by the study investigator. No specific criteria were included in the study protocols to exclude BOO. Post-residual urine volume was not measured in either study.
PRO measures common to both studies included the Patient Perception of Bladder Condition (PPBC), which measures the subject's perception of problems associated with their bladder condition [Citation12], and the Overactive Bladder Questionnaire (OAB-q), which assesses the level of bother and health-related quality of life (HRQL) impairment associated with their OAB symptoms [Citation13]. In VOLT, the primary efficacy endpoints were changes from baseline to Week 12 on the PPBC and symptom-specific visual analog scales. In VERSUS, the primary endpoint was improvement in diary-documented urgency episodes; the PPBC and OAB-q were secondary endpoints. The minimally important difference (MID), or the smallest numeric score change that subjects would perceive as beneficial, for all OAB-q scales and domains is 10 points [Citation14].
In VERSUS, OAB symptoms were recorded in 3-day bladder diaries, which were completed before Pre-Washout, Post-Washout, and at Weeks 4, 8, and 12. All post hoc comparisons were performed on data from men who received ≥1 dose of solifenacin and had baseline and ≥1 post-baseline efficacy assessment using 2-sided, 1-sample t tests at α = 0.05 (SAS; SAS Institute, Cary, NC).
Results
About 18% of VOLT and 12% of VERSUS participants were men. Compared with the men in VOLT, male subjects in VERSUS were older and a larger percentage was aged ≥75 years (). On average, men in VOLT weighed more and were more ethnically diverse than their VERSUS counterparts. At study entry for VOLT, 52% of men reported a history of urgency UI; in VERSUS, about 50% of men recorded UI in their baseline bladder diaries.
Table I. Baseline demographics.
At Week 4, 56% of men in VOLT and 65% of those in VERSUS increased their starting dose of 5 mg solifenacin to 10 mg; the majority of men in both studies continued taking 10 mg solifenacin through Week 8 (75% and 80%, respectively). In VOLT, an additional 23% of men who remained on 5 mg solifenacin at Week 4 increased to 10 mg at Week 8. None of the men in VERSUS increased their Week 4 dose of 5 mg to 10 mg, as per protocol.
At baseline, mean PPBC scores were similar for the male cohorts in VOLT (4.2) and VERSUS (4.0), corresponding to ‘some moderate’ bladder-related problems. After 12 weeks of solifenacin, PPBC scores decreased (i.e. improved) significantly among men in VOLT (−1.2, 95% CI −1.3 to −1.1; p < 0.001) and VERSUS (−0.7, 95% CI −1.1 to −0.3; p < 0.0001). At study end, PPBC scores in VOLT (3.0) and VERSUS (3.3) corresponded to ‘some minor’ bladder problems. In VOLT and VERSUS, 67% and 62% of men, respectively, showed improvement (≥1-point decrease) on the PPBC. As shown in , there was a clear shift toward fewer bladder-related problems (i.e. lower scores) after 12 weeks of solifenacin treatment in both studies. In both VOLT and VERSUS, men who received 12 weeks of solifenacin showed statistically significant improvements on all aspects of the OAB-q, including the Symptom Bother and HRQL scales and the HRQL domains (). All score changes reached or exceeded the 10-point MID. For diary-based endpoints collected during VERSUS, mean reductions from baseline to study end for daily episodes of urgency, frequency, UI, and nocturia were statistically significant among male subjects (). Relative (percentage) changes were greatest for UI, followed by urgency, nocturia, and frequency (). For all endpoints in both studies, results for the per-protocol set (PPS; data are not shown) were comparable to those reported above.
Figure 2. Distribution of male patients by PPBC score at baseline (VOLT, top) or Post-Washout (VERSUS, bottom) and end of treatment for both studies. PPBC, Patient Perception of Bladder Condition; VOLT, VESIcare Open-Label Trial; VERSUS, VESIcare Efficacy and Research Study US.
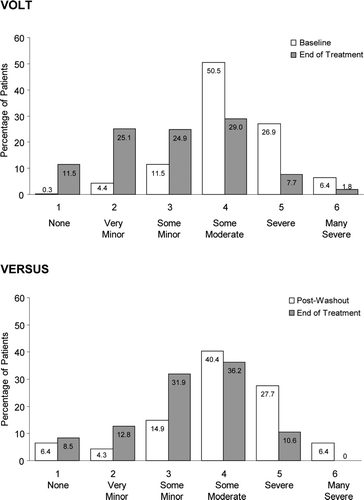
Figure 3. Change from baseline for OAB-q scores in men from VOLT and VERSUS. For the Symptom Bother scale, a negative value indicates improvement; for the HRQL scale and domains, a positive value indicates improvement. All changes from baseline were statistically significant (p < 0.001) as demonstrated using the two-sided, 1-sample t-test (α = 0.05). Scores shown from VERSUS are Post-Washout, after tolterodine cessation. HRQL, health-related quality of life; OAB-q, Overactive Bladder Questionnaire; VOLT, VESIcare Open-Label Trial; VERSUS, VESIcare Efficacy and Research Study US.
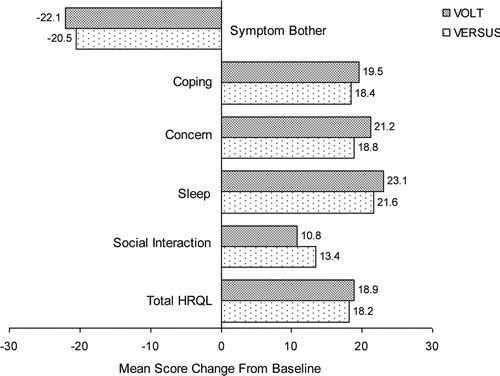
Figure 4. Results for diary-based endpoints in men from VERSUS: median percentage changes from baseline to study end (bar graph) and summary of mean values (table). BL, baseline; EOT, end of treatment; VERSUS, VESIcare Efficacy and Research Study US.
In both VOLT and VERSUS, the most frequently reported adverse events (AEs) were dry mouth and constipation (). Serious AEs were experienced by 3% and 8% of men in VOLT and VERSUS, respectively. In VOLT, the overall incidence of AUR was 0.05% (12/2,225); none of the cases occurred in men. In VERSUS, no cases of AUR were reported. In VOLT and VERSUS, 11% and 6% of men, respectively, discontinued solifenacin owing to AEs.
Table II. Treatment-emergent adverse events occurring in >1% of subjects in VOLT and VERSUS.
Discussion
Results from this post hoc analysis of data collected from men enrolled in two open-label studies of solifenacin showed that after 12 weeks of treatment, there were statistically significant improvements in PRO measures used to assess the impact of OAB on symptom-specific bother, HRQL, and overall bladder condition. We believe this report is the first to characterize the efficacy of antimuscarinic therapy in a large cohort of men with OAB using two validated, disease-specific questionnaires.
While an MID for the PPBC has not been established, men in VOLT and VERSUS showed significant reductions in mean PPBC scores, suggesting that their perception of their bladder condition improved from ‘some moderate’ to ‘some minor’ problems. The majority of men in VOLT and VERSUS showed improvement on the PPBC by study end. That there were significant changes on all aspects of the OAB-q suggests that solifenacin was efficacious in reducing the degree of bother associated with OAB symptoms in men who reported these symptoms at baseline. In fact, change scores on both scales and all domains reached or exceeded the MID of 10 points [Citation14], suggesting that the statistically significant numeric changes reflected clinically meaningful improvements in OAB symptom bother and aspects of HRQL among subjects who received solifenacin.
We identified only two studies that used subjective assessments and with which we can compare our PRO findings. Recently, Rovner et al. [Citation15] reported PRO results – including the OAB-q and PPBC – from TIMES (Tolterodine and Tamsulosin in Men with LUTS and OAB: Evaluation of Efficacy and Safety), a placebo-controlled trial of tolterodine ER with or without tamsulosin in men with OAB. On the OAB-q, the magnitude of changes in the tolterodine ER group was comparable with that observed with solifenacin in the VOLT and VERSUS studies. On the PPBC, most subjects in the tolterodine ER group showed improvement by Week 12 [Citation15]. In a 16-week, open-label study of Italian men, 5 mg solifenacin significantly improved mean PPBC score from baseline to study end [Citation16].
In VERSUS, treatment-related improvements in diary-documented urgency, incontinence, frequency, and nocturia were significant in this subpopulation of male patients. In the VESIcare Efficacy and Safety in Patients with Urgency Study (VENUS) [Citation17], a post hoc analysis showed that male patients also experienced marked changes versus placebo for daily episodes of urgency, frequency, incontinence, and nocturia. Our results are also consistent with similar post hoc evaluations of tolterodine ER for OAB in men. In a pooled analysis of data from two placebo-controlled trials, men who received tolterodine ER showed significantly greater mean reductions in 24-h micturitions versus those who received placebo [Citation18]. In a subanalysis of data from incontinent men, treatment with tolterodine ER was associated with significant mean reductions in UI episodes versus placebo [Citation19].
For all endpoints evaluated in VOLT and VERSUS, the results for the PPS were comparable to those reported above. This is not surprising, as the primary analysis for VOLT reported that treatment-related changes on the OAB-q and PPBC were comparable for the intent-to-treat (ITT) population and the PPS [Citation10]. For VERSUS, although the PPS results were not reported in the primary manuscript [Citation11], the findings for the OAB-q, PPBC, and diary variables were comparable to the ITT (data on file).
Tolerability profiles for men in VOLT and VERSUS were unremarkable, and in most cases, rates of the most frequently reported treatment-emergent AEs () were comparable with the full safety populations [Citation10,Citation11]. Interestingly, rates of dry mouth and constipation were lower in men from VERSUS than those from VOLT and also lower than rates for the full safety populations ().
No instances of AUR were reported among men in either study. This is encouraging, as men in VOLT and VERSUS were receiving flexibly dosed solifenacin (i.e. 5 or 10 mg). Ronchi et al. [Citation16] showed that after receiving 5 mg solifenacin for 16 weeks, only one male subject (2%) experienced AUR. Further, solifenacin-related changes in urodynamic parameters, including PVR and voided volume, were not clinically significant.
In VOLT and VERSUS, men with significant residual urines or suspected BOO were excluded. In both studies, most of the male patients were in their 60s. The exclusion of men with presumed BOO was pre-specified in the study protocol and decided by the study investigator, without the use of specific tests or measures. In the absence of pressure-flow urodynamics, we acknowledge that some men may have been obstructed. Still, there were no reports of AUR among the men in either study.
The results of a recent meta-analysis, designed to assess the efficacy and safety of anticholinergics for LUTS in men [Citation20], support our findings for VOLT and VERSUS. A literature search identified three relevant articles: an open-label study of men with urodynamically confirmed BOO and detrusor overactivity and symptoms refractory to alpha-blocker monotherapy [Citation21], and two randomized controlled trials of antimuscarinic monotherapy [Citation22,Citation23]. In the open-label study, Kaplan et al. [Citation21] investigated the efficacy of tolterodine monotherapy in men with alpha–blocker-refractory LUTS suggestive of BPH. After 6 months' treatment with tolterodine, improvements in urinary frequency and nocturia, Qmax and PVR volume, and AUA Symptom Index total and subscale scores were significantly improved. There were no reports of urinary retention in the open-label study.
The first controlled study to evaluate antimuscarinic therapy for LUTS used flavoxate versus placebo in men with symptomatic BPH [Citation23]. After 12 weeks, there were no statistically significant treatment differences for any endpoint, including diary- documented symptoms, subjective assessments of voiding and storage symptoms, and overall treatment response [Citation23]. In the second controlled study, Abrams et al. [Citation22] evaluated the safety of tolterodine versus placebo in men with OAB and urodynamically confirmed BOO in an equivalence study for Qmax. After 12 weeks, tolterodine significantly improved urodynamic parameters associated with storage symptoms without adversely affecting flow rate versus placebo. Only one placebo patient reported urinary retention. No subjective assessments were performed.
In the primary care setting, histological confirmations of BPH and pressure-flow studies to confirm BOO are not practical; however, other routine procedures and non-invasive urodynamics can be performed. It is essential to obtain a detailed history and determine the nature and severity of symptoms [Citation5,Citation24]. The American Urological Association Symptom Index (AUA-SI) and International Prostate Symptom Score (IPSS) are valid and reliable tools in this regard. Uroflowmetry can be used to determine maximum flow rate (Qmax) and PVR; a Qmax <10 ml/s is suggestive of BOO, and a PVR >50 ml is correlated with a higher risk of acute urinary retention [Citation24]. Finally, prostate size can be estimated via digital rectal examination, and prostate-specific antigen (PSA) level can be determined by blood tests.
Given that PSA levels are highly correlated with prostate volume [Citation25,Citation26], PSA measures alone can inform treatment decisions, and results from post hoc analyses of TIMES support this concept. In the subgroups of men with PSA levels <1.5 ng/ml [Citation27] or prostate volume <29 ml [Citation28] tolterodine ER monotherapy significantly improved frequency, frequency-urgency sum, urgency UI, and IPSS storage scores. In TIMES, PSA level also correlated significantly with prostate size [Citation28]. Together, the results of these assessments should be sufficient when making informed recommendations for first-line treatment of LUTS in men.
Nevertheless, several important considerations must be emphasized when treating male LUTS. Although symptoms and symptom bother dictate treatment initiation, they fail to differentiate between obstruction and OAB associated with aging or other causes. Other measures such as a high PVR may indicate obstruction, detrusor failure, or other problems that may require various interventions [Citation29]. High levels of PSA indicate larger prostate size, in which case voiding symptoms (such as a slow stream) are more likely to be associated with BPH, and treatment with α-blockers would be indicated. Because OAB symptoms are more likely to occur as part of LUTS with increasing age, the use of anticholinergic agents in unobstructed men may provide symptom relief and should be considered among the therapeutic options and interventions available to the clinician. Obviously, care should also be exercised in extremely frail or elderly men or those on multiple anticholinergics.
The strengths of this analysis are its inclusion of a large number of men with multiple, validated PRO measures and clinically utilized assessment for absence of substantial BOO. Weaknesses include the use of data from single-arm, open-label studies, which necessitates statistical testing against baseline values rather than a control group. The post hoc nature of the analysis also limits its statistical power. In the proper context, our results demonstrate the efficacy of solifenacin in a large cohort of presumably unobstructed men with OAB. These findings confirm and extend previous reports supporting a role for antimuscarinics in the treatment of bothersome symptoms among men with LUTS, particularly storage symptoms associated with OAB.
Acknowledgments
This study was undertaken with a research grant from Astellas Pharma US Inc. and GlaxoSmithKline. Editorial support, including writing assistance, was provided by Thomas Gegeny MS, ELS, and Linda A. Goldstein, PhD, medical writers at Envision Scientific Solutions, and was funded by Astellas Pharma Global Development Inc. and GlaxoSmithKline.
References
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 2002;21:167–178.
- Abrams P, Artibani W, Cardozo L, Dmochowski R, van Kerrebroeck P, Sand P. Reviewing the ICS 2002 terminology report: the ongoing debate. Neurourol Urodyn 2006;25:293.
- Chapple CR, Roehrborn CG. A shifted paradigm for the further understanding, evaluation, and treatment of lower urinary tract symptoms in men: focus on the bladder. Eur Urol 2006;49:651–659.
- Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, Coyne K, Kelleher C, Hampel C, Artibani W, Abrams P. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 2006;50:1306–1315.
- Miner MM. Primary care physician versus urologist: how does their medical management of LUTS associated with BPH differ? Curr Urol Rep 2009;10:254–260.
- Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol 2008;54:543–562.
- Khullar V, Chapple C, Gabriel Z, Dooley JA. The effects of antimuscarinics on health-related quality of life in overactive bladder: a systematic review and meta-analysis. Urology 2006;68(2 Suppl):38–48.
- Novara G, Galfano A, Ficarra V, Artibani W. Anticholinergic drugs in patients with bladder outlet obstruction and lower urinary tract symptoms: a systematic review. Eur Urol 2006;50:675–683.
- Kaplan SA. New data on tolterodine: do recent findings dispel questions about treating overactive bladder in men? Eur Urol Suppl 2007;6:10–16.
- Garely AD, Kaufman JM, Sand PK, Smith N, Masakazu A. Symptom bother and health-related quality of life outcomes following solifenacin treatment for overactive bladder: the VESIcare open-label trial (VOLT). Clin Ther 2006;28:1935–1946.
- Chancellor MB, Zinner N, Whitmore K, Kobashi K, Snyder JA, Siami P, Karram M, Laramee C, Capo JP, Jr., Seifeldin R, Forero-Schwanhaeuser S, Nandy I. Efficacy of solifenacin in patients previously treated with tolterodine extended release 4 mg: results of a 12-week, multicenter, open-label, flexible-dose study. Clin Ther 2008;30:1766–1781.
- Coyne KS, Matza LS, Kopp Z, Abrams P. The validation of the Patient Perception of Bladder Condition (PPBC): a single-item global measure for patients with overactive bladder. Eur Urol 2006;49:1079–1086.
- Coyne K, Revicki D, Hunt T, Corey R, Stewart W, Bentkover J, Kurth H, Abrams P. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res 2002;11:563–574.
- Coyne KS, Matza LS, Thompson CL, Kopp ZS, Khullar V. Determining the importance of change in the Overactive Bladder Questionnaire. J Urol 2006;176:627–632; discussion 632.
- Rovner ES, Kreder K, Sussman DO, Kaplan SA, Carlsson M, Bavendam T, Guan Z. Effect of tolterodine extended release with or without tamsulosin on measures of urgency and patient reported outcomes in men with lower urinary tract symptoms. J Urol 2008;180:1034–1041.
- Ronchi P, Gravina GL, Galatioto GP, Costa AM, Martella O, Vicentini C. Urodynamic parameters after solifenacin treatment in men with overactive bladder symptoms and detrusor underactivity. Neurourol Urodyn 2008;28:52–57.
- Karram MM, Toglia MR, Serels SR, Andoh M, Fakhoury A, Forero-Schwanhaeuser S. Treatment with solifenacin increases warning time and improves symptoms of overactive bladder: results from VENUS, a randomized, double-blind, placebo-controlled trial. Urology 2009;73:14–18.
- Dmochowski R, Abrams P, Marschall-Kehrel D, Wang JT, Guan Z. Efficacy and tolerability of tolterodine extended release in male and female patients with overactive bladder. Eur Urol 2007;51:1054–1064.
- Kaplan SA, Roehrborn CG, Dmochowski R, Rovner ES, Wang JT, Guan Z. Tolterodine extended release improves overactive bladder symptoms in men with overactive bladder and nocturia. Urology 2006;68:328–332.
- Gallegos PJ, Frazee LA. Anticholinergic therapy for lower urinary tract symptoms associated with benign prostatic hyperplasia. Pharmacotherapy 2008;28:356–365.
- Kaplan SA, Walmsley K, Te AE. Tolterodine extended release attenuates lower urinary tract symptoms in men with benign prostatic hyperplasia. J Urol 2005;174:2273–2275.
- Abrams P, Kaplan S, De Koning Gans HJ, Millard R. Safety and tolerability of tolterodine for the treatment of overactive bladder in men with bladder outlet obstruction. J Urol 2006;175(3 Part 1):999–1004.
- Dahm TL, Ostri P, Kristensen JK, Walter S, Frimodt-Moller C, Rasmussen RB, Nohr M, Alexander N. Flavoxate treatment of micturition disorders accompanying benign prostatic hypertrophy: a double-blind placebo-controlled multicenter investigation. Urol Int 1995;55:205–208.
- Kershen RT. Algorithms for the management of overactive bladder. Curr Urol Rep 2009;10:352–361.
- Lee SE, Chung JS, Han BK, Moon KH, Hwang SI, Lee HJ, Choe G, Hong SK. Relationship of prostate-specific antigen and prostate volume in Korean men with biopsy-proven benign prostatic hyperplasia. Urology 2008;71:395–398.
- Mao Q, Zheng X, Wang Y, Qin J, Yang K, Bai Y, Xie L. Relationships between total/free prostate-specific antigen and prostate volume in Chinese men with biopsy-proven benign prostatic hyperplasia. Int Urol Neph 2009 Feb 18 [Epub ahead of print].
- Roehrborn CG, Kaplan SA, Kraus SR, Wang JT, Bavendam T, Guan Z. Effects of serum PSA on efficacy of tolterodine extended release with or without tamsulosin in men with LUTS, including OAB. Urology 2008;72:1061–1067.
- Roehrborn CG, Kaplan SA, Jones JS, Wang JT, Bavendam T, Guan Z. Tolterodine extended release with or without tamsulosin in men with lower urinary tract symptoms including overactive bladder symptoms: effects of prostate size. Eur Urol 2009;55:472–479.
- MacDiarmid S, Rogers A. Male overactive bladder: the role of urodynamics and anticholinergics. Curr Urol Rep 2007;8:66–73.