Abstract
Objective. We examined baseline data from a lipid treatment study to assess the relationship between testosterone (T) and the cardiovascular inflammatory marker, high sensitivity C-reactive protein (hsCRP).
Methods. The baseline T, hsCRP, lipid, glycemic, and anthropometric data were obtained from 467 men (mean age: 52 years). Inclusion criteria included low-density lipoprotein cholesterol ≥ 3.4 to 4.9 mmol/l and triglycerides ≤ 4.0 mmol/l. The baseline hsCRP levels were examined across the following T subgroups: <6.9 nmol/l (moderate to severe hypogonadism), 6.9 to <10.4 nmol/l (mild to moderate hypogonadism), 10.4 to <15 nmol/l (low-normal T), and ≥ 15 nmol/l (normal T).
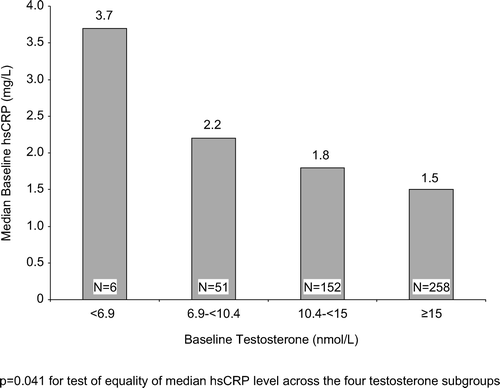
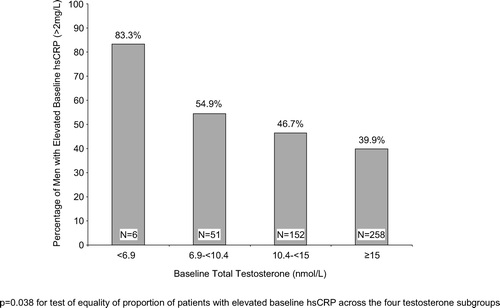
Results. The median hsCRP levels were significantly (p = 0.041) different across the four T subgroups; patients in the lower T subgroups had higher median hsCRP levels than patients in the higher T subgroups. The percentage of men with elevated hsCRP (>2 mg/l) was also significantly (p = 0.038) different across the four T subgroups; 83% of men with T < 6.9 nmol/l had elevated hsCRP compared with 40% with T ≥ 15 nmol/l.
Conclusions. This analysis demonstrated an inverse relationship between serum T and hsCRP in aging men. Urologists need to be aware that low T levels may not only adversely affect sexual function but also may worsen cardiovascular risk in aging, hypogonadal men.
Introduction
We had previously reported an analysis demonstrating an association between the metabolic syndrome and low total serum testosterone (T) levels in aging men using data from two lipid treatment studies [Citation1]. This previous analysis demonstrated that hypogonadism was significantly associated with three components of the metabolic syndrome in aging men, namely, hypertriglyceridemia, obesity, and the presence of high fasting plasma glucose (FPG)/diabetes [Citation1]. These data were in good agreement with the other studies that have demonstrated a relationship between hypogonadism and obesity, the metabolic syndrome and type 2 diabetes in aging men [Citation2–7].
One of the most commonly occurring symptoms associated with male hypogonadism is sexual dysfunction, leading a growing number of these patients to present at urology clinics. From a public health standpoint, it is important that urologists concerned with managing aging men with symptomatic hypogonadism understand the impact of low T levels not only on the sexual function of these patients but also on their cardiometabolic risk profile.
In addition to the metabolic syndrome and type 2 diabetes, low T has also been shown to be associated with elevated levels of the inflammatory marker, high sensitivity C-reactive protein (hsCRP), as well as other inflammatory markers, in men [Citation3,Citation8–10]. hsCRP has been demonstrated to be an independent cardiovascular risk marker – higher hsCRP levels are associated with greater cardiovascular risk in men and women [Citation11]. In light of these findings, we sought to determine the relationship between T and hsCRP in our aforementioned metabolic syndrome analysis patient cohort. One of the two lipid studies used in our previous analysis included hsCRP measurements – the present analysis evaluated baseline T and hsCRP data from men who participated in this study.
Methods
We examined the baseline total serum T and hsCRP data from 467 men (mean age: 52 years) who participated in a lipid treatment study. Inclusion criteria for this study included men and women aged 21–70 years with coronary heart disease (CHD) and/or atherosclerotic disease (AD) with low-density lipoprotein cholesterol (LDL-C) ≥ 3.4 mmol/l; or ≥ 2 CHD risk factors without CHD and/or AD with a LDL-C ≥ 4.1 mmol/l; or without CHD and/or AD and <2 risk factors with a LDL-C ≥ 4.9 mmol/l. Additional inclusion criteria included triglycerides (TG) ≤ 4.0 mmol/l. Exclusion criteria included a diagnosis of types I, III, IV, V hyperlipidemia or homozygous familial hypercholesterolemia; lipid-lowering agents taken within 6 weeks and fibrates taken within 8 weeks prior to screening; uncontrolled hypertension (treated or untreated) with systolic blood pressure >160 mmHg or diastolic blood pressure >100 mmHg; type 1 diabetes or type 2 diabetes with hemoglobin A1c ≥ 10%; and body weight >50% above or below ideal body weight according to the 1983 Metropolitan Height and Weight Tables [Citation12]. Plasma samples were assayed for hsCRP at a central laboratory using high-sensitivity immunonephelometry (Dade Behring, Deerfield, IL). The study protocol was approved by all relevant ethics review committees. Written informed consent was obtained from all patients prior to their participation in the study.
Statistical analysis
The baseline hsCRP levels were compared across the following T subgroups: <6.9 nmol/l (moderate to severe hypogonadism), 6.9 to <10.4 nmol/l (mild to moderate hypogonadism), 10.4 to <15 nmol/l (low-normal T), and ≥ 15 nmol/l (normal T). For each baseline T subgroup, median baseline hsCRP levels as well as the percentage of men with elevated hsCRP levels (>2 mg/l) were examined. Because hsCRP values are not normally distributed, the median was used for estimation of central tendency and a rank-based (Tukey's normal score) one-way ANOVA model was used for inferential testing. The equality of the proportion of patients with elevated hsCRP levels across the four T subgroups was tested using Fisher's exact test. Statistical significance was determined at the p <0.05 level.
Results
Baseline characteristics
provides a summary of the baseline characteristics of the men included in the present analysis. Compared to men in the higher baseline T subgroups, men in the lower baseline T subgroups had higher body mass indexes, higher TG levels, lower high-density lipoprotein cholesterol levels, higher FPG, and higher blood pressure. Additionally, the lower baseline T subgroups had a higher percentage of men with the metabolic syndrome compared to the higher baseline T subgroups.
Table I. Demographics and baseline characteristics.
Relationship between baseline total serum testosterone and high sensitivity C-reactive protein levels
shows median baseline hsCRP presented by baseline T level. Patients in the lower baseline T subgroups had higher median hsCRP levels than patients in the higher baseline T subgroups. The median hsCRP levels were significantly (p = 0.041) different across the four T subgroups. The percentage of men with elevated (>2 mg/l) baseline hsCRP was greater in the low T subgroups compared to the normal T subgroup (83% of men with baseline T < 6.9 nmol/l had elevated hsCRP compared with 40% of men with baseline T ≥ 15 nmol/l); . The percentages of men with elevated baseline hsCRP were significantly (p = 0.038) different across the four T subgroups.
Discussion
The findings reported in this analysis of baseline T and hsCRP data from a lipid treatment study are in good agreement with those of previous studies reporting an association between hypogonadism and elevated hsCRP levels as well as other inflammatory markers in aging men [Citation3,Citation8–10]. In the present analysis, there was a significant inverse relationship between median hsCRP levels and T, with median hsCRP being higher in men with hypogonadal T levels compared to men with normal T levels. Similarly, there was a significant inverse relationship between the percentage of men with elevated hsCRP (>2 mg/l) and T, with a higher percentage of men with hypogonadal T levels having elevated hsCRP compared to men with normal T levels. These data expand on the findings from our previous report demonstrating that hypogonadism was significantly associated with three of the five National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) [Citation13] components of the metabolic syndrome in aging men, namely, hypertriglyceridemia, obesity, and the presence of high FPG/diabetes [Citation1]. Each of these metabolic syndrome components has been shown to be associated with elevated hsCRP levels [Citation1,Citation14,Citation15]. Indeed, in the present study, the highest hsCRP levels were seen in those men with the highest occurrence of the five NCEP ATP III components of the metabolic syndrome. It therefore appears possible that the increase in hsCRP levels seen in hypogonadal men is being driven by the increased occurrence of these metabolic and anthropometric disorders.
The question arises as to whether T normalization could lead to a lowering of hsCRP levels in aging, hypogonadal men. T supplementation therapy has been shown to decrease visceral adiposity, FPG, and hypertriglyceridemia in aging men. Such effects would be expected to lead to a reduction in hsCRP levels, which may also be associated with a reduction in cardiovascular risk. Recent studies have demonstrated that long-term ( ≥ 6 months) T supplementation therapy consistently lowered hsCRP in aging, hypogonadal men [Citation16–18]. Additionally, administration of T in hypogonadal men was shown to result in decreased levels of tumor necrosis factor-α (TNF-α) and inteleukin-1β (IL-1β) without significant changes in IL-6, providing further support for an anti-inflammatory role for T [Citation19]. Whether or not such effects would be associated with a reduction in cardiovascular risk in these patients awaits determination in prospective cardiovascular outcomes trials.
In addition to T controlling inflammatory marker levels, it is also possible that inflammatory markers may play a role in controlling T levels. Thus, it is known that inflammatory cytokines inhibit Leydig cell function and decrease the sensitivity of Leydig cells to luteinizing hormone, which could result in a reduction in testicular output of T [Citation20].
One limitation of the current analysis concerns the relatively small sample size (n = 6) in the subgroup of men with severe hypogonadism. However, the significant trends seen in hsCRP levels across the T subgroups in our analysis are consistent with those reported in other studies [Citation3,Citation8].
In conclusion, this analysis of baseline data from a lipid treatment study showed an inverse relationship between total serum T and hsCRP levels in aging men. This negative relationship is likely driven by the increased occurrence of metabolic syndrome components in hypogonadal men, which are known to be associated with increased hsCRP levels. In light of the ongoing epidemic of obesity and the metabolic syndrome/type 2 diabetes in western countries [Citation21], and taking into account the association between these conditions/diseases and low T in aging men together with the current age range of the baby boomer population, it is likely that there will be a significant increase in the number of aging male patients presenting in urology clinics with symptomatic hypogonadism. Urologists need to be aware that low T levels may not only adversely affect sexual function but also may be associated with high hsCRP levels and heightened cardiometabolic risk in aging men. Further studies are needed to determine whether T supplementation therapy could lead to an improvement in cardiovascular outcomes in aging, hypogonadal men.
Acknowledgements
Dr. Kaplan has been an investigator in studies funded by Merck & Co., Inc. Drs. Johnson-Levonas, Shah, Meehan, and Mr. Lin are employees of and hold stock in Merck & Co., Inc. This study was funded by Merck & Co., Inc. The authors alone are responsible for the content and writing of the article.
References
- Kaplan SA, Meehan AG, Shah A. The age-related decline in testosterone is significantly exacerbated in obese men with the metabolic syndrome – what are the implications of this for the relatively high incidence of erectile dysfunction observed in these men? J Urol 2006;176:1524–1528.
- Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol 2002;57A:M76–M99.
- Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, Salonen R, Rauramaa R, Salonen JT. Sex hormones, inflammation and the metabolic syndrome: a population-based study. Eur J Endocrinol 2003;149:601–608.
- Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen T-P, Valkonen V-P, Salonen R, Salonen JT. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 2004;27:1036–1041.
- Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen T-P, Valkonen V-P, Salonen JT. The metabolic syndrome and smoking in relation to hypogonadism in middle-aged men: a prospective cohort study. J Clin Endocrinol Metab 2005;90:712–719.
- Pitteloud N, Mootha VK, Dwyer AA, Hardin M, Lee H, Eriksson K-F, Tripathy D, Yialamas M, Groop L, Elahi D, Hayes FJ. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care 2005;28:1636–1642.
- Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetics. J Clin Endocrinol Metab 2004;89:5462–5468.
- Bhatia V, Chaudhuri A, Tomar R, Dhindsa S, Ghanim H, Dandona P. Low testosterone and high C-reactive protein concentrations predict low hematocrit in type 2 diabetes. Diabetes Care 2006;29:2289–2294.
- Nakhai Pour HR, Grobbee DE, Muller M, van der Schouw YT. Association of endogenous sex hormone with C-reactive protein levels in middle-aged and elderly men. Clin Endocrinol 2007;66:394–398.
- Maggio M, Basaria S, Ble A, Lauretani F, Bandinelli S, Ceda GP, Valenti G, Ling SM, Ferrucci L. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab 2006;91:345–347.
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice. A statement for healthcare professionals from the Centers for Disease Control and prevention and the American Heart Association. Circulation 2003;107:499–511.
- Metropolitan Life Insurance Company. 1983. Metropolitan height and weight tables. Stat Bull 1983;64:2–9.
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497.
- Zuliani G, Volpato S, Galvani M, Blè A, Bandinelli S, Corsi AM, Lauretani F, Maggio M, Guralnik JM, Fellin R, Ferrucci L. Elevated C-reactive protein levels and metabolic syndrome in the elderly: the role of central obesity: data from the InChianti study. Atherosclerosis 2009;203:626–632.
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type diabetes: a systematic review and meta-analysis. JAMA 2006;295:1288–1299.
- Vertkin AL, Naumov AV, Morgunov LY, Kalinchenko SY, Krivtsova EV, Arinina EN, Kolosova ES, Plupanova YS. Testosterone effects on cardiovascular risk factors in men with metabolic syndrome. Cardiovasc Ther Prev 2008;7:68–75.
- Giltay EJ, Haider A, Saad F, Gooren LJ. C-reactive protein levels and ageing male symptoms in hypogonadal men treated with testosterone supplementation. Andrologia 2008;40:398–400.
- Haider A, Gooren LJ, Padungtod P, Saad F. Concurrent improvement of the metabolic syndrome and lower urinary tract symptoms upon normalisation of plasma testosterone levels in hypogonadal elderly men. Andrologia 2009;41:7–13.
- Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab 2004;89:3313–3318.
- Mauduit C, Gasnier F, Rey C, Chauvin M-A, Stocco DM, Louisot P, Benahmed M. TNF alfa inhibits leydig cell steroidogenesis through a decrease in steroidogenic acute regulatory protein expression. Endocrinology 1998;139:2863–2868.
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. JAMA 2002;287:356–359.