Abstract
Senescence is one of the main aetiological factors which are responsible for natural androgen ablation in men and occurrence of prostatic diseases. However, it is unclear how the prostatic lesions are signallised in the prostate. Thus, the aim of this study is to characterise the structural, the ultrastructural and the proliferative aspects of the peripheral prostatic zone in the elderly men with and without diagnoses of prostatic lesions and with potential precursors of prostate cancer. Sixty samples of prostatic tissue, from 60 to 90-year-old patients with and without lesions obtained from autopsied or prostatectomised patients were divided into four groups (15 samples per group): standard group (no lesions), benign prostatic hyper-plasia group, high-grade prostatic intra-epithelial neoplasia group and prostatic carcinoma group. The samples were submitted to morphometrical, structural and ultrastructural analyses in addition to cellular apoptosis and proliferative analyses. The results showed morphological damages in the stroma and cellular organelles involved in the secretory process of the prostate. Moreover, the prostatic lesions in elderly men demonstrated disturbance in the proliferation/apoptosis rate, indicating a prevalence of the proliferative process. Finally, the imbalance in prostatic stroma-epithelium interaction was a harmful feature in the elderly men as a result of structural changes, which are crucial factors for the development and progression of carcinogenesis.
Introduction
The prostate is a male accessory sex gland which is fundamental in the reproductive process [Citation1], showing epithelium with columnar secretory cells, and a layer of basal cells, the latter being closely related to the transport and distribution of substances between the epithelial and stromal compartments [Citation2]. Luminal epithelial cells are highly differentiated and represent the most frequent cell type both in normal and in hyper-plastic epithelium forming the prostatic exocrine compartments, which secrete proteins such as the prostate specific antigen (PSA) and prostatic acid phosphatase (PAP) [Citation3]. However, the basal cells are relatively undifferentiated, not showing secretory activity [Citation4].
The prostate stroma is made up of a complex arrangement of stromal cells and extracellular matrix associated to growth factors; regulatory molecules; and restructuring enzymes, which lead to general biological signs and have mechanical influences on the epithelial cells, apart from being considered an important morphogenesis and maturation regulator of the gland [Citation5]. The fibroblasts and smooth muscle cells are important cell types of the prostatic stroma, which synthesise structural and regulatory components of the extracellular matrix. The extracellular matrix is a network of fibrillar proteins; adhesive glycoproteins; and proteoglycans and is a reservoir of active and latent growth factors [Citation6]. Structural components such as collagen fibres and elastic fibres provide mechanical rigidity and flexibility to the tissue. Thus, stromal and extracellular matrix cell interaction create a microenvironment that regulates the growth and functional differentiation of adjacent cells [Citation7].
Prostate morphology and physiology has been analysed due to pathological conditions that affect this organ. Among these, benign prostatic hyperplasia (BPH), high-grade prostatic intra-epithelial neoplasia (HGPIN) and prostate cancer [Citation8] can be highlighted. BPH is characterised by predominant stromal proliferation, and although there is an increase of the epithelium, the regional integrity of the gland is preserved [Citation9]. On the other hand, prostate cancer is considered an epithelium disease and often extends itself beyond the normal limits of the organ [Citation9]. HGPIN is clinically pointed out as being a precursor lesion of invasive cancer; however, the molecular mechanisms that support this transition are not yet fully known [Citation10].
Senescence is one of the main aetiological factors responsible for natural androgen depletion in men, and occurrence of various prostatic diseases. However, it is not clear how the mechanism is signallised in the prostate.
Thus, the aim of this study is to characterise the structural, the ultrastructural and proliferative aspects of the peripheral prostatic zone in the elderly men with and without diagnoses of prostatic lesions and with potential precursors of prostate cancer.
Materials and methods
Human samples and tissue preparation
Sixty prostatic samples were obtained from 60 to 90-year-old patients (mean 63 years) with and without prostatic lesions hospitalised in the teaching Hospital of the School of Medicine, University of Campinas (UNICAMP). Fifteen prostatic samples were obtained from necropsied patients without diagnosis of prostatic or other urological diseases. The samples were obtained from the para-sagittal midline of the posterior surface of the prostatic peripheral zone. From these samples only areas with normal glands were included for the study.
In addition, another 45 prostatic samples were taken from the prostate of patients submitted to retropubic radical prostatectomy in a period from November 2008 to June 2009. The surgical specimens were whole-mount processed and totally embedded. A mean of 32 paraffin blocks (range 10 to 56 blocks) were processed, and 6 μm sections from each block were stained with hematoxylin and eosin. Areas of BPH, HGPIN and Gleason 3 + 3 = 6 prostatic carcinoma (PC) were diagnosed according to morphological criteria [Citation11] by a senior uropathologist (A. B.).
The prostatic samples were divided into four groups (15 samples per group): from the autopsied patients the standard group (no lesions) and from the patients submitted to prostatectomy the BPH Group, the HGPIN Group and the PC Group. The samples were submitted to morphometrical, structural, ultrastructural analyses in addition to cellular apoptosis and proliferative analyses.
Ethical permission was obtained from the Research Ethics Committee of the School of Medicine, University of Campinas/UNICAMP (number 0094.0.146.000-08).
Light microscopy
Prostatic samples from five patients in each group for histological analysis, fixed by immersion in Bouin's solution, embedded in paraplast (Paraplast Plus, Brazil), cut into 5-μm thick sections and submitted to the following staining procedures: Hematoxylin-Eosin [Citation12], Ammoniacal silver [Citation12], Masson's trichrome [Citation13] and Weigert's Resorcin-Fucsin. The slides were photographed with a Nikon Eclipse E-400 photomicroscope.
Transmission electron microscopy
Samples of the prostatic peripheral zone were collected from five patients in each group and were fixed with Karnovsky's solution. Next, the samples were immersed in cacodylate buffer and 1% osmium tetroxide for 1 h. After that, all the material was embedded in resin (Polysciences, Niles, IL), cut with an ultramicrotome (Ultracult UCT 020 Leica), mounted on copper grids (Sigma Chemical Company, St. Louis, MO), and counterstained with uranyl acetate and lead citrate. The specimens were then examined and photographed under a LEO 906 transmission electron microscope.
Morphometrical procedures
Prostatic samples, from five patients in each group were used for morphometrical analyses, the same used for light microscopy.
Cellular, cytoplasm and nuclear areas were measured (10 fields per sample were randomly taken), using 100× magnification. To determine epithelial and stromal areas, 10 fields per sample were randomly taken, using 20× magnification.
So as to determine autophagic and secretory vesicle areas, electron micrographs of five patients in each group, taken with a LEO 906 transmission electron microscope, were used. Ten cells from each sample per group were measured and the final magnification electron micrographs were measured using the following proportion: 3597 ×× 3.5 ×. The cells used were those which presented accurate limits, basal membrane and cellular apex.
The different parameters were measured using the NIS-elements: Advanced Research (Nikon, Tokyo, Japan) computerised image analysis system.
Detection of apoptosis and determination of the apoptotic index
Prostatic samples from five patients were fixed by immersion in 4% paraformaldehyde and processed for DNA fragmentation by means of a fluorescein apoptosis detection system (Promega, Madison, WI). The apoptotic nuclei were identified using a Nikon TS-100 microscope equipped for fluorescence (DXM 1200F, Nikon, Tokyo, Japan), in addition to Feulgen's reaction. The sections were subjected to hydrolysis with 4 N HCl for 75 min and then treated with Schiff's reagent for 40 min, for Feulgen's reaction. After washing, the sections were dehydrated and mounted on slides. Ten microscopic fields, with a total area of 90304.7 μm2, were randomly taken and analysed per sample, resulting in 50 fields per group, using a Nikon Eclipse E-400 photomicroscope with a 100× objective. The apoptotic index was determined by dividing the number of apoptotic nuclei by the total number of nuclei found in the microscope field. Apoptotic nuclei were identified by the characteristic pyknosis and/or nuclear fragmentation.
Immunolabelled Ki-67 and determination of the proliferative index
Prostatic samples from five patients, the same used for light microscopy, were taken and cut into 5 μm thick sections. The antigens were retrieved by boiling the sections in a 10 mM citrate buffer, pH 6.0, in a microwave oven. After that, the sections were incubated in 0.3% H2O2 for 15 min to block endogenous peroxidase. Non-specific binding was blocked by incubating the sections in a blocking solution for 1 h at room temperature. Primary rabbit AB-9260 (Chemicon International, USA) for the Ki-67 antibody was diluted in 1% BSA (1:50) and applied to the sections. The Envision HRP Kit (Dako Cytomation, Carpenteria, CA) was used to visualise the bound antibody. Secondary labelled polymer from the Envision HRP Kit (Dako) was applied for 40 min at room temperature. Peroxidase activity was detected using a diaminobenzidine chromogen kit from Envision HRP Kit (Dako) for 10 min. The sections were lightly counterstained with methyl green dehydrated in an increasing ethanol series and xylene, mounted in Entellan (Merck, Darmstadt, Germany) and photographed with a Nikon Eclipse E-400 photomicroscope. Each section was evaluated for the presence of brown DAB precipitate which indicates antibody binding.
The proliferative index was determined by Ki-67 immunolocalisation in five patients in different groups. Ten fields were taken at random and measured per sample, resulting in 50 fields per group with 100× magnification, representing a total area of 90304.7 μm2 and the total number of cells was expressed by means of percentage.
Statistical analyses
Mean data for cellular, nuclear, cytoplasmatic, epithelial, stromal areas and secretory and autophagic vesicle areas, apoptotic index and proliferative counting were compared among groups and analysed statistically by means of analysis of variance and Tukey's multiple range test, with the level of significance set at 5% [Citation14].
Results
Transmission electron and light microscopies and morphometrical analyses standard group (without structural changes)
The prostatic peripheral zone showed different sizes and folded mucosa of the acini (). The secretory epithelium presented a secretory layer of columnar cells and another one of basal cells ( and ). Flattened rough endoplasmic reticulum cisternae and developed Golgi complex in the supranuclear and perinuclear were observed in the cellular cytoplasm (). Also, secretory vesicles with granules, showing different electron density in the apical cytoplasm were presented (), representing a cytoplasmatic area which was 2.8 times greater than those found for digestory vacuoles (). The luminal surface had short and sparse microvilli (). The prostatic stroma showed thin collagen fibres underlying the secretory epithelium and intermingled with smooth muscle cells, and reticular and elastic fibres underlying the epithelium around the acini ( and ). The muscular layer presented long smooth fibres underlying the epithelium (). The stromal area was close to 6.7% greater than that found for the epithelial area ().
Figure 1. Photomicrographs of the prostatic peripheral zone from standard (a, b, c, d), HGPIN (e, f, g, h), PC (i, j, k, l) and BPH (m, n, o, p) groups. (a) Epithelium with secretory columnar and basal cells; inset: secretory epithelial cell nucleus (N), basal cells (arrow) with flattened nuclei. (b) Thin collagen fibres (Col) underlying the epithelium and among smooth muscle cells. (c), (d) Reticular (Rf) and elastic (EF) fibres underlying the epithelium. (e) HGPIN; inset: HGPIN focus, voluminous cellular nuclei (N) and basal cells (arrow). (f) Secretory epithelium with HGPIN foci (arrows), increased collagen fibres (Col) and smooth muscle cells. (g), (h) Increased reticular (Rf) and elastic (Ef) fibres. (i) Peaked neoplastic acini (asterisks) with infiltrative feature, periacinar space (arrows); Irregular smooth muscle cells; inset: neoplastic acini with increased nuclei (arrow). (j) Neoplastic acini (arrows); Increased collagen fibres (Col), smooth muscle fibres, amylaceous body (asterisk) and inflammatory cells (Ic). (k), (l) Disorganised and increased reticular (Rf) and elastic (Ef) fibres. (m) BPH with acinus showing an external straight line and internal papillomatosis. (n) Epithelium with secretory columnar and basal cells (arrow); hypercellular stroma with increased collagen fibres (Col). (o), (p) Hyper-plastic stroma with increased reticular (Rf) and elastic (Ef) fibres. a–p: Ep, epithelium, L, lumen, Smc, smooth muscle cell and St, stroma.
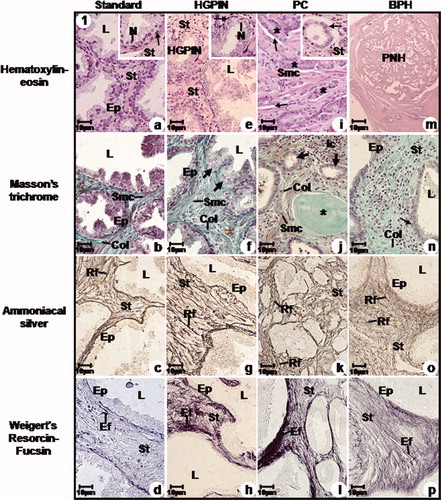
Figure 2. Electron micrographs of the prostatic peripheral zone from standard (a, b, c, d), HGPIN (e, f, g), PC (h, i, j) and BPH (k, l, m) groups. (a) Columnar cells with basal nuclei and clear nucleoli (Nu); Basal cells (Bc). Stroma (St). (b) Often secretory vesicles; short and scattered microvilli (Mv). (c) Flattened and parallel Golgi cisternae (arrow) with secretory vesicles. (d) Rough endoplasmic reticulum cisternae (RER). Basal lamina (arrow); Stroma with collagen fibres (Col) underlying the epithelium and among smooth muscle cells (Smc) and fibroblast (Fb). (e) HGPIN; Basal cells (Bc). (f) Dilated Golgi cisternae (arrow); inset: Ruptured mitochondrial cristae. (g) Dilated rough endoplasmic reticulum cisternae (RER). Digestory vacuoles (asterisk); Basal laminal (arrow); Increased collagen fibres (Col) distributed among smooth muscle cells (Smc). (h) Neoplastic cells with increased nuclei (arrow). (i) Neoplastic nuclei (arrow); amylaceous bodies (Ab); interrupted basal lamina (asterisk); Smooth muscle cells (Smc) showing irregular feature and collagen fibres (Col). (j) Neoplastic cell with increased nuclei and rare cytoplasm (arrow); Increased collagen fibres (Col). (k) Columnar cells. (l) Dilated rough endoplasmic reticulum cisternae (Rer) and Golgi (arrow). Digestory vacuoles (asterisk). (m) Basal cells (Bc); Digestory vacuoles (arrow) in the cellular cytoplasm; Increased collagen fibres (Col) and fibroblast (Fb) and smooth muscle cells (Smc); inset: smooth muscle cells showing spinous feature (Smc). a–m: L, lumen; M, mitochondria; N, nucleus; St, stroma and Vs, secretory vesicles.
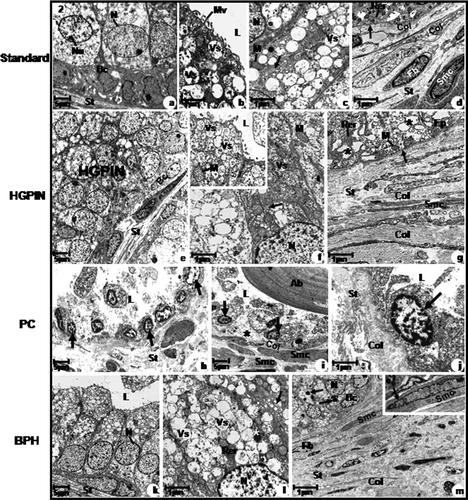
Table I. Mean and standard deviation of the nuclear, cytoplasmatic, epithelial, stromal, secretory vesicle (VS), digestory vacuoles (VD) areas (μm2) and percentage of apoptolic and proliferative index of the prostatic peripheral zone from different groups.
HGPIN group
HGPIN was verified in the prostatic peripheral zone, characterised by cytological atypia with voluminous and prominent nucleoli with different intensity ( and , ). Basal cells were identified ( and ). Occasional regions of dilated rough endoplasmic reticulum and Golgi complex cisternae were observed in the cellular cytoplasm (). The secretory vesicle area was 1.3 times greater than those found for digestory vacuoles; however this same area was significantly reduced if compared to the standard group (, ). Ruptured mitochondrial cristae were observed (), in addition to cellular cytoplasm vacuolisation (). Short and sparse microvilli covered the cellular surface.
Hypertrophied stroma with increased collagen fibres were verified, not only placed underlying the epithelium but also in the entire stromal area ( and ). The stromal area was close to 5.0 times greater than the epithelial area (). Increased reticular and elastic fibres showing ondulated form were verified in the all glandular stroma ().
PC group
The prostatic peripheral zone showed infiltrative adenocarcinoma characterised by peaked neoplastic acini in addition to a lack of basal layer and occurrence of periacinar spacing ( and ). The cell nuclei which covered the neoplastic acini were voluminous, showing an oval shape and prominent nucleoli, however the nuclear area was significantly smaller than that found in the HGPIN group and greater than those found in the standard and BPH groups (, and ). Occasional organelles and secretory vesicles were observed in the cellular cytoplasm (). The secretory vesicle areas were significantly smaller than those found for HGPIN, standard and BPH (). The digestory vesicles showed an inverse relation when compared to secretory vesicles (). Amylaceous bodies were verified in the neoplastic acinus lumen ( and ). The basal lamina showed discontinuous regions (). Lack of microvilli was verified in the cellular surface ().
Moderate stromal hypertrophy was observed with an amount of reticular, elastic and collagen fibres close to those found for the standard group ( and ). The stromal area increased in relation to the epithelial one (). Nevertheless, this rate increased quantitively in comparison to the standard group and was significantly smaller in relation to HGPIN and BPH groups (). The smooth muscle fibres were dispersed and with spinous features ( and ). Also, the occurrence of inflammatory cells in the stroma was verified ().
BPH group
Well limited hyper-plastic nodules were verified in the peripheral zone, characterised by acinus showing an external straight line and internal papillomatosis ().
The secretory epithelium presented tall columnar cells with basal nuclei and clear nucleoli in addition to basal cell layer ( and ). The nuclear area was greater, quantitively in relation to the standard group and significantly smaller in comparison to the HGPIN and PC groups (). Dilated rough endoplasmic reticulum and Golgi cisternae were verified (). The secretory vesicle area was not significantly different from that found for the HGPIN group, however this same area was smaller than that found for the standard group and greater than that found for the PC group ( and ). On the other hand, the digestory vacuole area was greater than that found for the HGPIN and standard groups, in terms of number and significantly smaller than that found for the PC group ( and ). Rare microvilli covered the cellular surface ().
Hyper-plastic and hyper-cellular stroma with a great amount of collagen, reticular and elastic fibres were observed both underlying the epithelium and intermingled with the smooth muscle cells ( and ). Increased stromal area was verified in relation to the epithelial area (). Folded sarcolemma was seen in the smooth muscle cells, characterising spinous features ().
Detection of apoptosis and determination of the apoptotic index
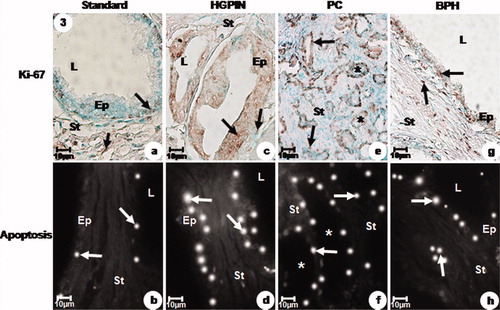
The apoptotic index significantly increased in the HGPIN and BPH groups in relation to standard and PC groups (). Nevertheless, the PC group showed an average apoptotic index which was significantly greater than those found for the standard group ( and ).
Figure 3. Immunolabelled Ki-67 and apoptotic index of the prostatic peripheral zone from standard (a, b), HGPIN (c, d), PC (e, f) and BPH (g, h) groups. (a) Weak Ki-67 immunoreactivity (arrows) in the secretory epithelial and stromal cells. (b) Weak DNA fragmentation (arrows) in the secretory epithelial cells. (c) Intense Ki-67 immunoreactivity (arrows) in the secretory epithelial and stromal cells. (d) Intense DNA fragmentation (arrows) in the secretory epithelial cells. (e) Intense Ki-67 immunoreactivity (arrows) in the neoplastic acini (asterisks) and stromal cells. (f) Moderate DNA fragmentation (arrows) in the neoplastic acini (asterisks). (g) Moderate Ki-67 immunoreactivity (arrows) in the secretory epithelial and stromal cells. (h) Moderate DNA fragmentation (arrows) in the secretory epithelial cells. a–h: Ep, epithelium, L, lumen and St, stroma.
Immunolabelled Ki-67 and determination of the proliferative index
The proliferative response was significantly greater in HGPIN than those found for PC, BPH and standard groups, which showed decreasing values for this variable ( and ).
Discussion
The study herewith demonstrated that the prostatic stroma occupied the great majority of the glandular area in elderly men. In addition, in the elderly men who had characterised prostatic diseases the changes was much intensified, specially, in the HGPIN and PC groups. The group with HGPIN showed cytological atypia characterised by large nuclei and prominent nucleoli, presence of basal layer of epithelial cells, which were responsible for the integrity of the architectural arrangement and basal membrane. A decrease of secretory vesicles in relation to normal and hyper-plastic prostate tissue as well as an increase in the proliferative process could also be seen. However, the PC group showed absence of basal layer and basal membrane rupture points, apart from strong cellular atypia and architectural disorder. The BPH group was characterised by hypercellularity and increased fibrillar elements in the stroma as well as an increase of proliferation. Also, other morphological changes which were in common such as disorganisation of organelles involved in the secretory process; stromal hyperplasia; presence of inflammatory cells in the prostatic stroma; smooth muscle cell with secretory phenotype; and increased apoptotic process were identified in the HGPIN, PC and BPH groups.
HGPIN is an abnormal differentiation and proliferation process, so that the layer of basal cells loses its proliferation capacity, which is carried out by the luminal secretory cells, characterising typical pre-malignant disorder [Citation15]. All genotypic and phenotypic information showed HGPIN to be a precursor lesion of invasive adenocarcinoma [Citation16].
In adenocarcinoma, the loss of basal cell differentiation is a critical point in the progression of invasive cancer and is probably related to the inability of transformed cells to produce basal membrane proteins [Citation17]. The causal association of HGPIN with adenocarcinoma is based on the fact that their prevalence increases as the patient gets older and that HGPIN may precede the onset of prostate cancer [Citation16].
The prostatic stroma is a fundamental compartment in the function of the gland because of its role in maintaining the prostate homeostasis and its morphological and physiological involvement in diseases such as BPH and cancer [Citation18]. On the basis of morphological, functional and embryology aspects, epithelium–stromal interaction may be considered as a single functional unit [Citation19]. The alterations in the normal homeostatic stromal–epithelial interactions may play a role in the pathogenesis of the prostate [Citation20]. The alterations of stromal microenvironment are the initial steps in the development and progression of prostate cancer [Citation21]. The increase in the production of extracellular matrix, especially collagen, growth factors and reorganisation of stromal components is known as reactive stroma, creating a microenvironment which can cause tumour growth [Citation22]. Reactive stroma alters the stromal–epithelium interaction benefiting the expansion of genetically altered epithelial cells [Citation23]. An event that frequently occurs during carcinogenesis is the change in stromal cell composition, which involves the replacement of smooth muscle cells with a fibroblast population, due to changes in the differentiation of muscle cells or the recruitment of fibroblasts to tumour location [Citation21]. Different authors observed altered phenotype from smooth muscle cells in remodelling tissues, in response to various injuries; and in prostate carcinomas [Citation24]. Thus, not only the hypertrophy of the muscle layer and the synthetic phenotype acquired by smooth muscle cells, but also the accumulation of collagen, suggest that these cells have an active role in the constant remodelling of the extracellular matrix that is possibly occurring in the stroma of the prostatic peripheral areas in senile men with HGPIN, PC and BPH.
Although proliferation and apoptosis processes were greater due to the prostate lesions, the proliferative process was greater representing a 4.2 time increase compared to a 2.3 time increase of the apoptosis process. Also, it was found that the ratio between proliferation and apoptosis was 1.4 in elderly healthy men and 2.0 in men with BPH, 2.6 in men with HGPIN and 3.0 in men with PC.
Apoptosis is a physiological active cellular mechanism, which occurs during the processes of development and restructuring tissue [Citation25]. This is characterised by morphological changes that include loss of plasma membrane and condensation of the cytoplasm and the nucleus [Citation26]. This mechanism ensures the physiological organisation of tissue in the development and cellular balance of the organ in adults and is often associated with the process of tissue involution [Citation27]. Thus, the mechanism is the balance between proliferation and cell death [Citation26]. The cell survival of many tissues depends on a constant supply of growth factors and/or hormones. The prostate is an androgen-regulated gland. The lack of testosterone leads to rapid tissue involution due to apoptotic elimination of glandular cells [Citation28]. In hormone-dependent tumours, such as prostate cancer, the induction of programmed cell death due to androgen ablation has been associated with tumour regression [Citation29].
Age is indicated as a cumulative event due to the interaction between intrinsic and extrinsic factors in the development of hyperplasia. Intrinsic factors such as extracellular matrix, smooth muscle and epithelial cells, as well as environmental factors, are known to influence the differentiation and morphogenesis of the prostate gland [Citation30].
The blocking of cell replication classified by senescence is identified as being a possible intrinsic mechanism in the defence against epithelial cancer. In this mechanism, the cells stop replication, but they remain metabolically active. The change from senescent cells influences adjacent cells, such as what happens to senescent fibroblasts, which interfere in the proliferation increase and tumourigenicity of epithelial cells [Citation6]. These changes in the architecture of the tissue in the senescence have been considered as a potential factor to harmful effects in the prostate. Paradoxically, although there is evidence that the aging cell can develop mechanisms to prevent malignant transformation by blocking the replication of carcinogenic events [Citation31], new studies suggest that senescent cells can alter the glandular microenvironment, promoting carcinogenesis in non-senescent epithelium [Citation6]. Thus, the aging first inhibits the development of cancer, but later senescent cells change the microenvironment causing epithelial cancer [Citation32].
Therefore, it can be concluded that the prostatic lesions in elderly men demonstrated disturbance in the proliferation/apoptosis rate, indicating a prevalence of the proliferative process. Moreover, it can also be seen that the imbalance in prostatic stroma–epithelium interaction was a harmful feature in the elderly men as a result of structural changes, which are crucial factors for the development and progression of carcinogenesis. Finally, it can also be said that these structural changes have distinct intensities in BPH, HGPIN and prostatic cancer.
The structural changes observed in senescence as well as in all the prostatic diseases verified in elderly men, such as HGPIN, nodular hyperplasia and adenocarcinoma effectively jeopardised the cellular organelles involved in the secretory process of the organ.
Acknowledgements
This work was supported by the state of São Paulo Research Foundation (FAPESP 2008/53468-1), National Council for Scientific and Technological Development (CNPQ 300068/2008-5).
References
- Cavazos F. Fine structure and functional correlates of male accessory sex glands of rodents. In: Greep RO, Astwood EB. Handbook of physiology. Washington (DC): American Physiological Society, 1975:353–381.
- Garraway LA, Lin D, Signoretti S, Waltregny D, Dilks J, Bhattacharya N, Loda M. Intermediate basal cells of the prostate: in vitro and in vivo characterization. The Prostate 2003;55:206–218.
- Taylor RA, Risbridger GP. The path toward identifying prostatic stem cells. Differentiation 2008;76:671–668.
- Mcneal JE. Normal histology of the prostate. Am J Surg Pathol 1988;12:619–633.
- Dean JP, Nelson PS. Profiling influences of senescent and aged fibroblasts on prostate carcinogenesis. Br J Cancer 2008;98:245–249.
- Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol 2001;166:2472–2483.
- Cornell RJ, Rowley D, Wheeler T, Ali N, Ayala G. Neuroepithelial interactions in prostate cancer are enhanced in the presence of prostatic stroma. Urology 2003;61:870–875.
- Leav I, Lau KM, Adams JY, McNeal JE, Taplin ME, Wang J, Singh H, Ho SM. Comparative studies of the estrogen receptors β and α and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol 2001;159:79–92.
- Droller MJ. Medical approaches in the management of prostatic disease. Br J Urol 1997;79:42–52.
- Majumder PK, Grisanzio C, O'Connell F, Barry M, Brito JM, Xu Q, Guney I, Berger R, Herman P, Bikoff R, et al A prostatic intraepithelial neoplasia-dependent p27Kip1 checkpoint induces senescence and inhibits cell proliferation and cancer progression. Cancer Cell 2008;14:146–155.
- Mostofi FK, Price EB Jr. Tumors of the male genital system, atlas of tumor pathology, 2nd Series, Fascicle 8. Washington (DC): Armed Forces Institute of Pathology, 1973:202–217.
- Behmer OA, Tolosa EMC, Freitas-Neto AG. Manual para histologia normal e patológica. Edart-Edusp, 1976, São Paulo, 225.
- Junqueira LCU, Bignolas G, Brentani R. Picrossirius staining plus polarization microscopy, specific method of collagen detection in tissue section. J Histochem 1979;11:447–455.
- Zar HA, Tanigawa K, Kim YM, Lancaster JR. Mild therapeutic hypothermia for postischemic vasoconstriction in the perfused rat liver. Anesthesiology 1999;90:1103–1111.
- Bonkhoff H, Stein U, Remberger K. The proliferative function of basal cells in the normal and hyperplastic human prostate. Prostate 1994;24:114–118.
- Bostwick DG, Liu L, Brawer MK, Qian J. High-grade prostatic intraepithelial neoplasia. Rev Urol 2004;6:171–179.
- Bonkhoff H, Fixemer T, Rememberg K. Relation between Bcl-2, cell proliferation, and the androgen receptor status in prostate tissue and precursors of prostate cancer. Prostate 1998;34:251–258.
- Zhang Y, Nojima S, Nakayama H, Jin Y, Enza H. Characteristics of normal stromal components and their correlation with cancer occurrence in human prostate. Oncol Rep 2003;10:207–211.
- Hayward SW, Cunha GR. The prostate: development and physiology. Radiol Clin N Am. 2000;38:1–14.
- Cunha GR, Hayward SW, Wang YZ. Role of stroma in carcinogenesis of the prostate. Differentiation 2002;70:473–485.
- Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer 2003;107:1–10.
- Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res 2002;8:2912–2923.
- Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD, Sudilovsky D, Cunha GR. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res 2001;61:8135–8142.
- Fávaro WJ, Cagnon VH. Morphometric and morphological features of the ventral prostate in rats submitted to chronic nicotine and alcohol treatment. Tissue Cell 2006;38:311–323.
- Kerr M, Lee A, Wang PL. Detection of insulin and insulin-like growth factors I and Iiin saliva and potential synthesis in the salivary glands of mice. Biochem Pharmacol 1995;49:1521–1523.
- Sohn BH, Moon HB, Kim TY, Kang HS, Bae YS, Lee KK, Kim SJ. Interleukin-10 up-regulates tumour-necrosis-factor-α-related apoptosis-inducing ligand (TRAIL) gene expression in mammary epithelial cells at the involution stage. Biochem J 2001;360:31–38.
- Rauch F, Polzar B, Stephan H, Zanotti S, Paddenberg R, Mannherz HG. Androgen ablation leads to an upregulation and intranuclear accumulation of deoxyribonuclease I in rat prostate epithelial cells paralleling their apoptotic elimination. J Cell Biol 1997 ; 137:909–923.
- Kyprianou N, Isaacs JT. Activation of programmed cell death in the rat ventral prostate after castration. Endrocrinology 1988;122:552–562.
- Staack A, Kassis AP, Olshen A, Wang Y, Wu D, Carroll PR, Grossfeld GD, Cunha GR, Hayward SW. Quantitation of apoptotic activity following castration in human prostatic tissue in vivo. Prostate 2003;54:212–219.
- Zenzmaier C, Untergasser G, Berger P. Aging of the prostate epithelial stem/progenitor cell. Exp Gerontol 2008;43:981–985.
- Parrinello S, Coppe JP, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci 2005;118:485–496.
- Sprenger CC, Plymate SR, Reed MJ. Extracellular influences on tumour angiogenesis in the aged host. Br J Cancer 2008;98:250–255.
