Abstract
Introduction. Patients with late onset hypogonadism (LOH) also suffered from lower urinary tract symptoms (LUTS) and LOH symptoms. The objects of this study are to evaluate the efficacy of testosterone replace therapy (TRT) by testosterone ointment (Glowmin: GL) for LUTS in LOH patients.
Methods. The Aging Male Symptom (AMS) scale, Medical Outcomes Study (MOS) 36-Item Short-Form Health Survey (SF-36), International Index of Erectile Function (IIEF-5) and the International Prostate Symptom Score (IPSS) were obtained from patients with LOH. A total of 41 patients with LOH have been treated with TRT using 6 mg/day of GL for 3 months. Serum free testosterone levels (FT) and these four scores were compared before and after TRT.
Results. Serum FT levels and the scores for the four parameters of AMS, six of eight domains in SF-36, IIEF-5 and total IPSS improved significantly after 3 months TRT. In addition, all IPSS domains also improved significantly, and voiding disturbance seems to have improved more than storage disturbance (P = 0.0280 vs. 0.0483).
Conclusion. TRT by administration of GL is considered to be effective in the improvement of not only ED and LOH symptoms, but also LUTS (especially voiding disturbance) of patients with LOH.
Introduction
Late-onset hypogonadism (LOH) is defined as the condition caused by the decline of testosterone by aging, along with various symptoms, including physical, psychological and sexual disturbance [Citation1]. Testosterone replacement therapy (TRT) has been applied primarily to alleviate the various symptoms in patients with LOH [Citation2–5]. It has also reported that TRT using testosterone ointment (Glowmin: GL) appeared to improve those LOH symptoms [Citation6].
Recently, the relationship between erectile dysfunction (ED) and lower urinary tract symptoms (LUTS) has been discussed. The statistical relations and the common mechanism of both ED and LUTS have been reported [Citation7–11].
Patients with LOH suffer from various symptoms, including ED, thus LUTS conditions should be considered in patients with LOH. It has already been demonstrated that patients with LOH also suffered from LUTS and LOH symptoms [Citation12].
We compared LUTS scores obtained by IPSS before and after TRT. The objects of this study were to evaluate the efficacy of TRT by testosterone ointment (GL) for LUTS in patients with LOH.
Methods
According to the Japanese LOH guideline [Citation13], normal range of serum free testosterone (FT) level in Japanese men is greater than 11.8 pg/ml. The Aging Male Symptoms (AMS) [Citation14] score is widely accepted for assessing and monitoring the symptoms of LOH, and the diagnosis of LOH is not made for patients with an AMS score of 17–26. Thus, the patients with low serum FT levels (<11.8 pg/ml), and a high AMS score (>26) were enrolled in this study.
Forty-one patients with LOH who agreed to participate in this pilot trial and had signed in informed consent were treated with testosterone ointment (GL) for 3 months. GL (Daito Pharmaceutrical, Tokyo, Japan) contains 10 mg of testosterone per 1 g of matrix (1%). A 4-cm line of GL was applied to the lower abdominal skin and contained a dosage of 6 mg of testosterone and this was given once a day in the morning.
Serum FT levels were measured before and after TRT. The AMS score, International Index of Erectile Function (IIEF-5) [Citation15], Medical Outcomes Study (MOS) 36-Item Short-Form Health Survey (SF-36) [Citation16] and International Prostate Symptom Score (IPSS) were obtained from these 41 patients with LOH. To evaluate the efficacy of TRT in patients with LOH, these four scores (AMS, IIEF-5, SF-36 and IPSS) were compared before and after TRT. Furthermore, to clarify which LUTS symptoms that TRT has more effect on, IPSS and its sub-scores (post-voiding, storage, voiding symptoms and QOL) were also analysed before and after TRT.
Statistical analysis
Paired t-test was performed to compare several parameters before and 3 months after TRT, assuming P < 0.05 as significant.
Results
Ages of the 41 patients with LOH ranged from 31 to 75 (54.9 ± 9.8) years. The mean FT levels of these patients prior to GL treatment was 6.8 ± 1.5 (4.0–11.3) pg/ml. AMS score ranged from 35 to 75 (53.2 ± 9.4) before GL treatment. All these patients indicated lower FT levels (Japanese LOH criteria [Citation13] and higher AMS score [Citation14].
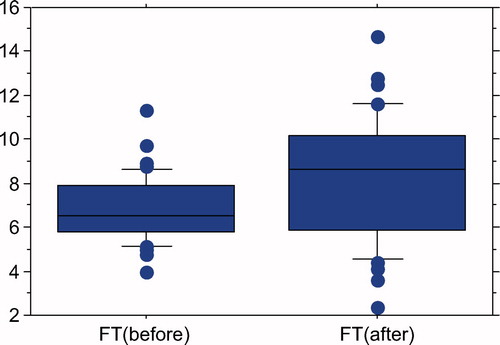
Three months after 6 mg/day of testosterone ointment therapy with GL, serum FT levels have significantly risen from 6.82 ± 1.51 to 8.24 ± 2.84 pg/ml (P = 0.0047; paired t-test) ().
Figure 1. Serum FT levels of 41 patients with LOH. FT levels significantly elevated from 6.82 ± 1.51 to 8.24 ± 2.84 pg/ml after 3 months TRT with GL 6 mg/day (P = 0.0047; paired t-test).
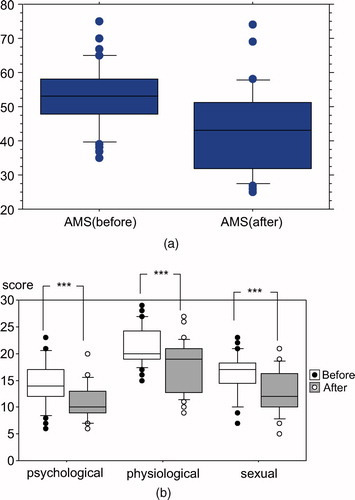
Total AMS scores had also decreased from 53.2 ± 9.4 to 42.7 ± 12.2 by GL administration (). All three domains of AMS, including psychological, physiological and sexual disturbance had been significantly reduced (P < 0.001; paired t-test) ().
Figure 2. (a) Total AMS score significantly improved from 53.2 ± 9.4 to 42.7 ± 12.2 by TRT (P<0.0001; paired t-test). (b) All three domains of AMS (psychological, physiological and sexual) were relieved after TRT (all P < 0.0001; paired t-test).
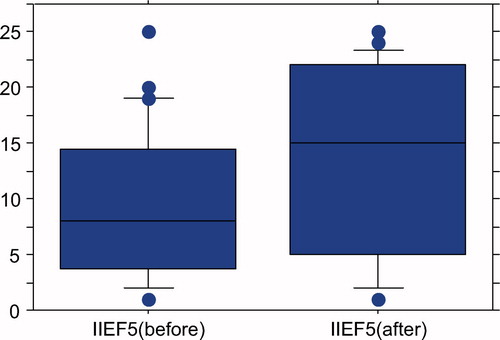
IIEF-5 scores as the parameter of ED had significantly risen from 9.2 ± 6.6 to 13.4 ± 8.3 after 3 months GL treatments (P = 0.0001; Paired t-test) ().
Figure 3. IIEF-5 scores have significantly raised from 9.2 ± 6.6 to 13.4 ± 8.3 after 3 months GL application (P = 0.0001; paired t-test).
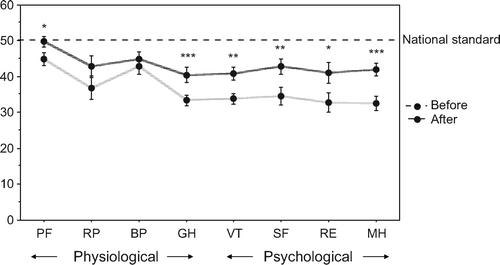
All eight domains of SF-36 (physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE) and mental health (MH)) levels were lower than average before TRT. Of the eight domains of SF-36, six of them, PF (P < 0.01), GH (P < 0.0001), VT (P < 0.005), SF (P < 0.005), RE (P < 0.05) and MH (P < 0.0005) significantly improved after 3 months TRT, although they were still below average levels (). Psychological bothers of SF-36 were much more improved compared with physiological bothers in LOH patients treated with GL.
Figure 4. In SF-36 domains, physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE) and mental health (MH)) levels were lower than average before TRT. After 3 months TRT, PF (P < 0.01), GH (P < 0.0001), VT (P < 0.005), SF (P < 0.005), RE (P < 0.05) and MH (P < 0.0005) data showed significant improvement.
Total IPSS score of the 41 patients with LOH before TRT ranged from 0 to 25 (8.5 ± 6.5); this meant many of them suffered from sight to moderate LUTS symptoms. After 3 months GL treatments, the total IPSS score significantly decreased to 6.0 ± 5.4 (0–28) (P = 0.0086; paired t-test) ().
Table I. All parameters of total IPSS, post-voiding, storage, voiding and QOL were improved by TRT.
All sub-scores of IPSS, including post-voiding (IPSS question 1), storage (IPSS question 2, 4, 7), voiding symptoms (IPSS question 3, 5, 6) and QOL (QPL question) improved after TRT treatments by GL. The concrete value of each score and P-value with paired t-test is shown in . All LUTS symptoms improved after TRT, and the voiding function has improved more from TRT compared with the storage function (P = 0.0280 vs. 0.483).
Discussion
Patients with LOH complain and suffer from various symptoms, including physical, psychological and sexual disturbance [Citation1]. In a previous report, 121 patients with LOH were also bothered by LUTS especially having voiding symptoms. Furthermore, age and AMS score were found to be important factors that influenced LUTS severity [Citation12].
The primary treatment for LOH in Japan is TRT with an intramuscular injection of testosterone enanthate [Citation13]. Different testosterone replacements are widely used in many other countries; however, these testosterone replacements have been not permitted by the Japanese Food and Drug Administration (FDA). Testosterone ointment (GL) is one of the approved agents for TRT for patients with LOH in Japan [Citation6]. GL contains 10 mg testosterone per 1 g (1%) and is an OTC (over the counter) ointment that was approved by the Japanese FDA in 1965. The efficacy of GL to alleviate many LOH symptoms has been reported [Citation6]. In this study, we investigated LUTS in patients with LOH and assessing the typical LOH symptoms, and the IPSS change after TRT by application of GL.
The pre-treatments baseline of 41 patients revealed low FT, high AMS scores, moderate to severe ED and slight to moderate LUTS. The QOL levels determined by SF-36 were not as good compared with the average level. After 3 months TRT using GL, serum FT levels, AMS scores, 6 domains of SF-36 and IIEF-5 scores had significantly improved, though they were not yet within normal range. Because GL is short acting there was only a mild elevation of FT levels that was not beyond the physiological level. Therefore, the improvement of FT, AMS scores, SF-36 and IIEF-5 scores was not as dramatic, but in fact rather mild.
Nevertheless, according to the results of the IPSS scores in this study, the patients also admitted an improvement of LUTS. The efficacy of TRT for LUTS in patients with LOH is still controversial. Karazindiyanoglu et al. [Citation17] reported that TRT with transdermal testosterone 50–100 mg gel per day for 1 year alleviated LUTS in 25 patients with LOH. TRT with either testosterone gel (50 mg) daily or injections of testosterone undecanoate 1000 mg also improved many parameters, including testosterone levels, IPSS, AMS scale, IIEF, C-reactive protein (CRP) and so on in LOH men [Citation18,Citation19]. On the other hand, Takao et al. [Citation20] reported that the efficacy of TRT by testosterone or hCG injection was suspicious. In this preliminary study, the LUTS in patients with LOH have been relieved with TRT by GL ointment. There is a possibility that the difference of efficacy for LUTS might be based on the difference of TRT dugs and/or application route. GL is a short acting ointment and the elevation of serum testosterone levels observed was within normal range. This physiological effect of GL may have some favourable effects on LUTS.
The results observed from this pilot study may indicate an additional common mechanism and treatment with TRT for not only LOH but also LUTS. There are several common mechanisms of ED and LUTS reported such as the autonomic nervous system, arteriosclerosis and pelvic ischaemia and Rho-kinase theory [Citation21]. Furthermore, the more common treatments for ED and LUTS have been reported such as PDE 5 inhibitors and alpha-blockers. PDE 5 inhibitors affect urethral and bladder smooth muscle resulting in improvement of male LUTS [Citation22,Citation23] and alpha-blocker may also improve ED [Citation24–28]. Although several explanations have been considered [Citation29], the mechanism of testosterone on LUTS in elderly men is not elucidated yet [Citation18,Citation19].
There are two main aspects in patients with LUTS: voiding and storage symptoms. Most of epidemiologic studies have not distinguished between voiding and storage symptoms in assessing the relationship between LUTS and ED [Citation30]. Some reports have shown that the relationship between ED and voiding symptoms are dominant [Citation31,Citation32], others have indicated that ED and storage symptoms are associated [Citation30–35]. A previous report showed that of 121 patients with LOH they also suffered from LUTS, especially voiding symptoms [Citation12]. This study data revealed that the voiding disturbance have improved, and that the voiding symptoms seems to be more correlated with LOH symptoms.
Testosterone affects many organs in the human body and improves several symptoms for patients with LOH. However, it is not known whether the improvement of LUTS is due to TRT affecting the lower urinary organs directly, or the relief of several symptoms secondary resulting in LUTS improvement. Although the mechanism of TRT on LUTS is not clear yet, our data supported that TRT had the possibility to improve LUTS in patients with LOH. We have speculated from this study looking at the SF-36 data that there may be a psychological influence. Psychological relief that is often reported after TRT also can have an effect on the improvement of several LOH symptoms, including LUTS.
TRT and its effect on the prostate is a very important issue. The analysis of a systematic review indicated that testosterone therapy did not have a consistent effect on prostate-specific antigen (PSA) levels [Citation36]. It has also been reported that no change in PSA levels were observed after 3 months GL treatments [Citation6]. Therefore, the prostate cancer risk is considered to be minimal. Furthermore, the prostate volume did not increase after TRT [Citation37] or even slightly increase [Citation17,Citation38], but voiding dysfunction was not reported.
GL is an OTC ointment, and no placebo is available for research purposes, due to the disallowance of placebo products by the Japanese government. Therefore, although it would have been far preferable to perform a placebo controlled study, currently this is not feasible given the current restrictions. In addition, the presenting patients for this study were patients with LOH, and they had not complained of any urinary disturbance. Therefore, the normal further investigations for their voiding dysfunctions, such as uroflowmetry and urodynamic studies, were not performed. However, this is a pilot study and it has shown LUTS in patients with LOH were alleviated after administration of TRT using GL ointment. It certainly would be considered advantageous that the treatment for LOH patients had additional positive effects on LUTS. This data does open the door to further investigate the relationship between LUTS and LOH/ED.
Conclusion
This study has demonstrated that TRT positively affected not only LOH symptoms, but also LUTS in patients with LOH. Although further studies including a placebo controlled trial would supplement this research, TRT by GL is an acceptable treatment to relieve LUTS in patients with LOH.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- NieschlagE, SwerdloffR, BehreHM, GoorenLJ, KaufmanJM, LegrosJJ, LunenfeldB, MorleyJE, SchulmanC, WangC, Investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male 2005;8:56–58.
- MoralesA, TenoverJL. Androgen deficiency in the aging male: when, who, and how to investigate and treat. Urol Clin N Am 2002;29:975–982.
- LunenfeldB, SaadF, HoeslCE. ISA, ISSAM and EAU recommendations for the investigation, treatment and monitoring of late-onset hypogonadism in males: scientific background and rationale. Aging Male 2005;8:59–74.
- LunenfeldB, NieschlagE. Testosterone therapy in the aging male. Aging Male 2007;10:139–153.
- WangCM, NieschlagE, SwerdloffRS, BehreH, HellstromWJ, GoorenLJ, KaufmanJM, LegrosJJ, LunenfeldB, MoralesA, ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male 2009;12:5–12.
- AmanoT, ImaoT, TakemaeK, IwamotoT, YamakawaK, BabaK, NakanomeM, SugimoriH, TanakaT, YoshidaK, Profile of serum testosterone levels after application of testosterone ointment (Glowmin) and its clinical efficacy in late-onset hypogonadism patients. J Sex Med 2008;5:1727–1736.
- ShiriR, HakkinenJT, HakamaM, HuhtalaH, AuvinenA, TammelaTL. Koskimaki J. Effect of lower urinary tract symptoms on the incidence of erectile dysfunction. J Urol 2005;174:205–209.
- El-SakkaAI. Lower urinary tract symptoms in patients with erectile dysfunction: analysis of risk factors. J Sex Med 2006;3:144–149.
- RhodenEL, RiednerCE, FornariA, FuchsSC, RibeiroER. Evaluation of the association between lower urinary tract symptoms and erectile dysfunction, considering its multiple risk factors. J Sex Med 2008;5:2662–2668.
- MorantS, BloomfieldG, VatsV, ChappleC. Increased sexual dysfunction in men with storage and voiding lower urinary tract symptoms. J Sex Med 2009;6:1103–1110.
- MariappanP, ChongWL. Prevalence and correlations of lower urinary tract symptoms, erectile dysfunction and incontinence in men from a multiethnic Asian population: Results of a regional population-based survey and comparison with industrialized nations. BJU Int 2006;98:1264–1268.
- AmanoT, ImaoT, TakemaeK. Lower urinary tract symptom in patients with late-onset hypogonadism. Jpn J Impotence Res 2009;24:359–365.
- Japan Urological Association/Japan Men's Health Medical Association. Clinical guideline for late-onset hypogonadism (LOH). Tokyo, Japan: KK Jiho; 2007. pp 2–28.
- HeinemannLAJ, ZimmermannT, VermeulenA, ThielC, Hummel. A new ‘aging males’ symptoms' rating scale. Aging Male 1999;2:105–114.
- RosenRC, CappelleriJC, SmithMD, LipskyJ, PenaBM. Development and evaluation of an abridged, 5-item version of the international index of erectile function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999;11:319–326.
- FukuharaS, WareJE, KosinskiM, WadaS, GandekB. Psychometric and clinical tests of validity of the Japanese SF-36 Healthy Survey. J Clin Epidemiol 1998;51:1045–1053.
- KarazindiyanogluS, CayanS. The effect of testosterone therapy on lower urinary tract symptoms/bladder and sexual functions in men with symptomatic late-onset hypogonadism. Aging Male 2008;11:146–149.
- KalinchenkoS, VishnevskyEL, KovalAN, MskhalayaGJ, SaadF. Beneficial effects of testosterone administration on symptoms of the lower urinary tract in men with late-onset hypogonadism: a pilot study. Aging Male 2008;11:57–61.
- HaiderA, GoorenLJ, PadungtodP, SaadF. Concurrent improvement of the metabolic syndrome and lower urinary tract symptoms upon normalization of plasma testosterone levels in hypogonadal elderly men. Andrologia 2009;41:7–13.
- TakaoT, TsujimuraA, NakayamaJ, MatsuokaM, MiyagawaY, TakadaS, NonomuraN, OkuyamaA. Lower urinary tract symptoms after hormone replacement therapy in Japanese patients with late-onset hypogonadism: a preliminary report. Int J Urol 2009;16:212–214.
- McVaryK. Lower urinary tract symptoms and sexual dysfunction: epidemiology and pathophysiology. BJU Int 2006;97 (suppl 2):23–28.
- TinelH, Stelte-LudwigB, HutterJ, SandnerP. Pre-clinical evidence for the use of phosphodiesterase-5 inhibitors for treating benign prostatic hyperplasia and lower urinary tract symptoms. BJI Int 2006;98:1259–1263.
- MatsumotoS, HanaiT, UemuraH, LevinRM. Effects of chronic treatment with Vardenafil, a phosphodiesterase 5 inhibitor, on female rat bladder in a partial bladder outlet obstruction model. BJU Int 2009;103:987–990.
- SairamK, KulinskayaE, McnicholasTA, BousteadGB, HanburyDC. Sildenafil influences lower urinary tract symptoms. BJU Int 2002;90:836–839.
- MulhallJP, GuhringP, ParkerM, HoppsC. Assessment of the impact of sildenafil citrate on lower urinary tract symptoms in men with erectile dysfunction. J Sex Med 2006;3:662–667.
- GacciM, Del PopoloG, MacchiarellaA, CelsoM, VittoriG, LapiniA, SerniS, SandnerP, MaggiM, CariniM. Vardenafil improves urodynamic parameters in men with spinal cord injury: results from a single dose, pilot study. J Urol 2007;178:2040–2044.
- McVaryKT, RoehrbornCG, KaminetskyJC, AuerbachSM, WachsB, YoungJM, EslerA, SidesGD, DenesBS. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol 2007;177:1401–1407.
- LiguoriG, TrombettaC, De GiorgiG, PomaraG, MaioG, VecchioD, OcelloG, OllandiniG, BucciS, BelgranoE. Efficacy and safety of combined oral therapy with tadalafil and alfuzosin: an integrated approach to the management of patients with lower urinary tract symptoms and erectile dysfunction. Preliminary report. J Sex Med 2009; 6:544–552.
- YassinAA, El-SakkaAI, SaadF, GoorenLJG. Lower urinary-tract symptoms and testosterone in elderly men. World J Urol 2008;26:359–364.
- IrwinDE, MilsomI, ReillyK, HunskaarS, KoppZ, HerschomS, CoyneKS, KelleherCJ, ArtibaniW, AbramsP. Overactive bladder is associated with erectile dysfunction and reduced sexual quality of life in men. J Sex Med 2008;5:2904–2910.
- BraunMH, SommerF, HauptG, MathersMJ, ReifenrathB, EngelmannUH. Lower urinary tract symptoms and erectile dysfunction: co-morbidity or typical “Aging Male” symptoms? Results of the “Cologne Male Survey”. Eur Urol 2003;44:588–594.
- PonholzerA, TemmlC, ObermayrR, MadersbacherS. Association between lower urinary tract symptoms and erectile dysfunction. Urology 2004;64:772–776.
- FrankelSJ, DonovanJL, PetersTI, AbramsP, DabhoiwalaNF, OsawaD, LinAT. Sexual dysfunction in men with lower urinary tract symptoms. J Clin Epidemiol 1998;51:677–685.
- AslanG, CavusE, KarasH, OnerO, DuranF, EsenA. Association between lower urinary tract symptoms and erectile dysfunction. Arch Androl 2006;52:155–162.
- LeliefeldHH, StoevelaarHJ, McDonnellJ. Sexual function before and after various treatments for symptomatic benign prostate hyperplasia. BJU Int 2002;89:208–213.
- ShabsighR, CrawfordED, NehraA, SlawinKM. Testosterone therapy in hypogonadal men and potential prostate cancer risk: a systematic review. Int J Impot Res 2009;21:9–23.
- TenoverJS. Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab 1992;75:1092–1098.
- HolmangS, MarinP, LindstedtG, HedelinH. Effect of long-term oral testosterone undecanoate treatment on prostate volume and serum prostate-specific antigen concentration in eugonadal middle-aged men. Prostate 1993;23:99–106.