Abstract
Context. In aging men, circulating testosterone (T) declines which is associated with an increase in the levels of luteinizing hormone (LH) and follicle stimulating hormone (FSH) , albeit insufficient to maintain T at its original level. It has been speculated that a higher sensitivity of the hypothalamus and/or pituitary for the feedback effect of circulating sex hormones in older men is responsible.
Objective. To compare the effect of experimentally varied plasma levels of estradiol on the LH and FSH secretion in young and old castrated male-to-female transsexuals, in almost absence of T.
Design, subjects, and interventions. In 10 healthy, young (mean age 37.6 ± 6.2 years) and 11 healthy, old (mean age 68.1 ± 7.0) male-to-female transsexuals after gonadectomy plasma estradiol levels were experimentally varied with estradiol patches (the first week 100 μg/day patches, the second week 50 μg/day, the third week 25 μg/day and the fourth week no patch was applied) and plasma levels of LH and FSH were monitored after every week.
Results. Mean plasma bioavailable estradiol (E2) levels in the two groups ranged between 13.6 and 104 pmol/l. LH and FSH were inversely related to peripheral estradiol levels, were lower in the old group at all time points reaching statistical significance in the last week of the study when no patch was applied and estradiol levels were extremely low.
Conclusions. The results of this study do not support the hypothesis of an age related increasing sensitivity of the hypothalamo-pituitary compartment for the negative feedback of E2, but suggest a deficient feed-forward drive in older male-to-female transsexuals.
Keywords:
Introduction
Aging in men is associated with a gradual declining mean level of circulating testosterone (T) [Citation1–6]. After the age of 60 year, more than 20% of men has a serum T level below the lower limit of the reference range for young males [Citation1,Citation7]. With aging, luteinizing hormone (LH) levels tend to increase [Citation8,Citation9], however, in a large number of older men this compensation does not appear to prevent the observed decline in T levels [Citation10,Citation11].
Several explanations have been suggested [Citation12]; decreased secretion of hypothalamic gonadotropin releasing hormone (GnRH), decreased testicular responsiveness to the stimulatory action of LH [Citation13–17] and an increased feedback sensitivity of the hypothalamus and/or the pituitary to the feedback effects of testicular androgens in older men [Citation18–22]. Consistent with this hypothesis, observations of Winters et al. [Citation23] showed that suppression of LH secretion during T treatment is greater as men age.
In an earlier study, we presented evidence that circulating estradiol also has an inhibitory effect on gonadotropin release [Citation24]. This contribution of estradiol in the negative feedback regulation of gonadotropin secretion in men has been well established [Citation25–29]. Therefore, the postulated increased age-related feedback sensitivity for the feedback of androgens might also reflect an increased sensitivity for the feedback of estrogens. To gain more insight into the effects of aging on gonadotropin release we chose an experimental model in which; (1) T, estradiol and inhibin levels are extremely low so that the unopposed production of gonadotropins can be studied in old and young man, (2) estradiol levels are clamped so that the response of gonadotropins to varying estradiol levels can be compared between old and young men. Our hypotheses are that (1) unopposed gonadotropin production is lower in older men and (2) the older hypothalamus and/or pituitary is more sensitive to the feedback effects of estradiol.
Subjects and methods
Subjects
The subjects were 21 apparently healthy male-to-female transsexuals, after gonadectomy. In the Netherlands, the treatment of genderdysforia is highly institutionalised and restricted to experienced academic centres. Before hormonal and surgical treatment subjects undergo intensive psychological evaluation to firmly establish a diagnosis of genderdysforia and identify physical, psychological and social risk factors that may complicate treatment. During treatment, patients are physically and psychologically monitored according to the recommendations of the WPATH (www.wpath.org). We aimed to create a group of younger individuals (age <45 years) and a group of older individuals (age > 60 years). Eventually, the young group consisted of 10 subjects between the ages of 22–44 year (mean age 37.6) and the old group consisted of 11 subjects between the ages of 60–80 year (mean age 68.1). Mean body mass index (BMI) of the young group was 25.7 (range 16.9–33.6) kg/m2 and of the old group 26.8 (range 19.1–36.1) kg/m2. In the young group, castration took place 6.8 years (range 1–17) before the start of the study and in the old group 17.5 years (range 1–35). Subjects were substituted with either oral or transdermal estradiol after surgery according to our postsurgery protocol. This protocol allows for a maximal prescribed daily dose of 4 mg oral estradiolvalerate or 100 μg transdermal estradiol. Subjects above the age of 40 are routinely advised to use transdermal instead of oral estradiol in order to minimise the risk of thrombosis. In the young group, eight subject (80%) used oral estradiol 2 mg per day and 2 subjects transdermal estradiol 100 μg per day, in the old age group 6 (54.5%) used oral estradiol 2 mg per day, and 5 used transdermal estradiol 100 μg per day. To prevent very high gonadotropin levels at the start of the study, subjects were immediately switched to study medication without a washout period. Exclusion criteria were a history of thrombosis and hyperprolactinemia. The results of the physical examination were within normal limits, except for appropriate surgical adaptations to obtain the desired female characteristics (genital surgery and breast augmentation).
Study protocol
All volunteers gave written informed consent before the start of the study and the study was approved by the Medical Ethics Committee of the Amsterdam VU University Medical Centre. All subjects were asked to stop their prescribed hormonal substitution. During week 1–3 estradiol was administrated using estradiol patches (Dermestril; Rottafarm, Italy) in decreasing doses. The dose of the patches was 100, 50 and 25 μg estradiol per day, respectively. During the 4th week no patch was applied. Patches were applied according to the recommendations of the manufacturer. Patches were replaced on days 4 and 7 of each treatment week or earlier whenever more than 25% of the patch was detached. Blood samples were collected at the end of every study week by venipuncture between 0800 and 1100 h to determine LH, follicle stimulating hormone (FSH) T, estradiol and sex hormone binding globuline (SHBG). Blood samples were allowed to clot and, after centrifugation, serum was stored at −20°C until analysis.
Hormone analysis
Levels of LH, FSH and SHBG were estimated by luminescence-based immuno-assays using an Immulite 2000 (Diagnostic Products Corp., Los Angeles, CA). Inter- and intra-assay coefficients of variation were less than 4.9, 5.9 and 6.3%, respectively. T and estradiol were measured using Coat-a-Count RIA obtained from the same supplier. Variation coefficients for the T assay were less than 7.5%. For the estradiol assay, variation coefficients were 11.8% for levels between 8 and 87 pmol/l and less than 9.8% for higher concentrations. The lowest detectable level, defined as blank values plus three standard deviation of the blank, was 7 pmol/l. Finally, cross-reactions were 10% for estrone, 1.8% for estrone glucuronide and less than 0.6% for all other naturally occurring steroids tested. Male reference values for these assays were 50–200 pmol/l for estradiol, 1.5–8 IU/l for LH, 2–7 IU/l for FSH, 10–30 nmol/l for T, 10–70 nmol/l for SHBG. Bioavailable estradiol was calculated using the method previously described by Sodergard et al. [Citation30] with a fixed albumin level of 4.3 g/l. The constants used in the analysis for the binding of E2 to albumin is 4.21 × 10E4 l/mol and for E2 to SHBG 3.14 × 10E8 l/mol.
Statistics
Mean levels for the analysed hormones and SHBG were calculated, grouped by study week and by study group. For baseline characteristics and hormone levels, differences between groups were tested for significance using a Students t-test for independent samples. For hormone levels obtained during the study, differences within and between groups were tested for significance using a general linear model for repeated measurements with the study week as the independent variable (within subjects factor), total estradiol, bioavailable estradiol, LH, FSH and SHBG as dependent variables and the age group as a between subject factor. Levels of LH and FSH were logarithmically transformed to achieve a linear relationship with the independent variable. When the sphericity assumption was not met, the Huynh–Feldt correction was applied. BMI and time since castration were added to the model as covariates.
Results
Hormone and SHBG levels at baseline are summarised in . The time of castration was significantly different between the age groups (p = 0.001). Because of the difference between the age groups in use of estradiol supplement (80% oral in the young group vs. 54.5% in the old group), we also compared the two groups of estradiol use, oral intake vs. transdermal application, for hormone and SHBG levels, summarised in . E2 levels, but also LH, FSH and SHBG levels tended to be lower in the transdermal group, however not reaching statistical significance.
Table I. Parameters at baseline, comparing the age group.
Table II. Parameters at baseline, comparing estradiol use.
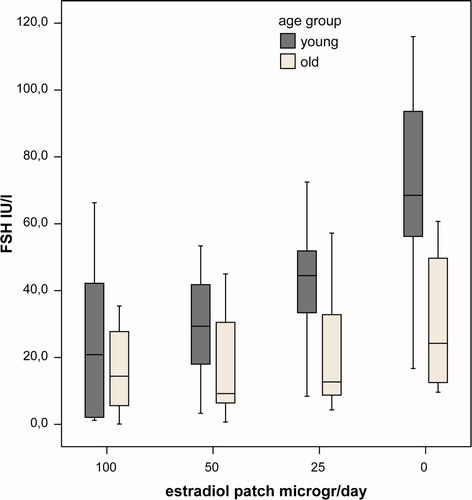
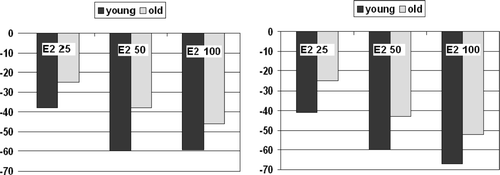
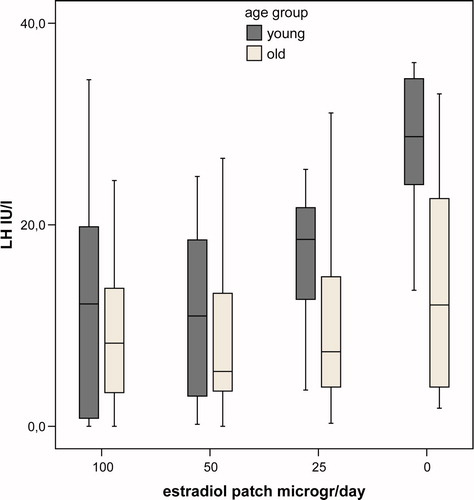
Hormone and SHBG levels during the study are summarised in and , representing the differences in levels between the age groups per week of the study. In the last week of the study, mean levels of bioavailable E2 were very low and not significantly different between groups (p = 0.85). In this week mean levels of LH and FSH were significantly lower in the old group (p = 0.02, and p = 0.004, respectively). During the study there was a dose-dependent decrease of (bioavailable) E2 levels during estrogen application. Mean bioavailable E2 levels per dose were higher at all time points in the young group, but this difference did not reach statistical significance (p = 0.17 for between subjects effect. Mean levels of SHBG were not significantly different between groups (p = 0.37 for between subjects effect), but the mean SHBG levels decreased significantly during the study (p = 0.04). Levels of LH and FSH mirrored the fluctuations in peripheral estradiol levels. For all time points the mean levels of LH and FSH were higher in the young group, but due to the large overlap between groups the difference did not reach statistical significance (p = 0.09 and 0.06, respectively, for between subjects effect). In none of the models, age group, BMI, different hormone therapy before study or time since castration significantly interacted with the relationship between bioavailable E2 and gonadotropin levels. In , the suppressing effect of E2 on gonadotropin levels in young and old individuals are depicted. The suppressive effect of estradiol was not significantly different between young and old male-to-female transsexuals (p = 0.17 and p = 0.11 for between subjects effects for LH and FSH, respectively).
Table III. Hormone and SHBG levels in studied subjects (mean ± SD), grouped by study week and age group.
Figure 1. Box plot of bioavailable estradiol in young (black) and old (grey) castrated men per study week.
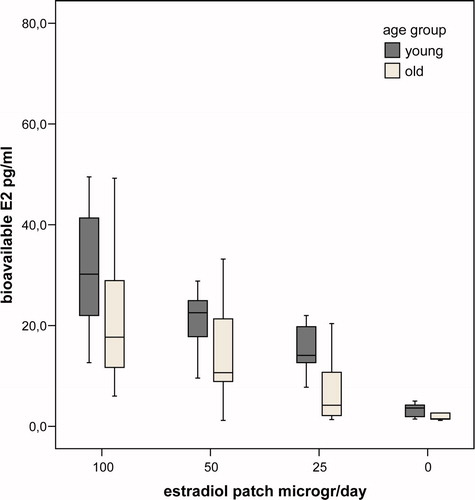
Figure 2. Box plot of LH levels in young (black) and old (grey) castrated men per study week. Significant difference between the age groups only in the week when no patch was applied (p = 0.02).
Discussion
In the present study, we monitored the effects of circulating estradiol concentrations on the levels of LH and FSH in castrated old and young biological males (male–female transsexuals). We experimentally varied circulating E2 levels and compared the gonadotropin response in young and old individuals to evaluate the role of aging in the estradiol mediated inhibition of LH and FSH release. When T and E2 levels were extremely low, young subjects had higher levels of LH and FSH compared to old subjects. This indicates that the gonadotropin production is reduced in older male-to-female transsexuals when the feedback regulating hormones estradiol and T are extremely low, although there is considerable overlap between old and young individuals. Lower gonadotropin levels may result from reduced GnRH production or reduced responsiveness of the pituitary to the stimulatory effect of GnRH. Taking into consideration that most experimental evidence revealed that pituitary responsiveness is maintained with aging [Citation16,Citation31,Citation32], our observations are consistent with the previously mentioned hypothesis that GnRH outflow is reduced in older male-to-female transsexuals, independent of the postulated altered sensitivity for the feedback effects of circulating sex hormones. The exact mechanisms underlying the decrease in hypothalamic GnRH secretion in elderly male-to-female transsexuals remain to be elucidated. Postulated mechanisms in elderly men include alterations in GnRH pulsefrequency and/or an impaired access of GnRH to the anterior-pituitary gonadotroph cells. Disordered LH-pulse patterns have been observed in aging men [Citation33,Citation34], with an increased LH pulse frequency but with a reduction of the mean LH pulse amplitude and amount of large amplitude LH pulses [Citation10]. This suggests a diminution of the integrity of the feed-forward system, which might be the consequence of a reduced number of functional hypothalamic GnRH neurons, intrinsic changes in the GnRH neurons and/or an inadequate coordinated recruitment of GnRH neurons, needed for an adequate GnRH bolus release.
Our study showed that circulating E2 has a substantial effect on the levels of LH and FSH, which illustrates the important contribution of estrogens to the feedback inhibition of gonadotropin secretion in male-to-female transsexuals [Citation24,Citation35,Citation36].
Although increasing E2 reduced gonadotropin levels in both age groups, age did not significantly alter the relationship between E2 and gonadotropin levels. These observations do not support the hypothesis that gonadotropin secretion is more sensitive to the suppressive effects of circulating E2 in older men. This is concordant with the observations of T'Sjoen et al. [Citation37], who showed that the rise in gonadotropin levels in response to aromatase inhibition was similar in young and elderly men. Also in the study by Winters et al. [Citation21], the suppressive effect of infused estradiol on gonadotropins was similar in older compared to young men. However, in the same study older men responded with a greater inhibition of gonadotropin release than young men on T and dihydrotestosterone infusions. This suggests that gonadotropin secretion in aging men is more sensitive to inhibition by androgens but not estrogens.
Limitations of the presents study are the relatively small number of subjects and a limited amount of blood samples, taken only once weekly. Therefore, we did not obtain information about possible changes in the gonadotropin pulse frequency and amplitude. There was also a significant difference between the groups regarding the time of castration before the start of the study. It might be speculated that prolonged treatment with estrogens results in permanent alterations in the hypothalamo-pituitary compartment resulting in altered gonadotropin release during or after alleviation of estrogen supplementation. This concept is supported by observation of a different (female-like) LH response to GnRH stimulation and pulsatile estrogen administration in males before and during long-term estrogen treatment [Citation38]. In our study, a dose dependent inverse association between estradiol and gonadotropin levels was seen in both young and older individuals, consistent with a male-type suppressive effect of estradiol on LH and FSH release. In a case report by Wortsman et al. [Citation39], hypogonadotrophic hypogonadism was observed after prolonged treatment with DES, which is a synthetic, non steroidal estrogen, and a causal relationship was suggested albeit not proven. In our study, all subjects are and have been treated with sex hormone replacement therapy since their castration. The time since castration did not emerge as an independent determinant of gonadotropin response. Therefore, the observed differences between young and older individuals are more likely to be due to their age rather than to the duration of estrogen treatment. However, it cannot be excluded that the altered hormonal milieu has had lasting effects on gonadotropin secretion and therefore extrapolation of our findings to normal men should be done with caution.
There was a difference in hormonal replacement therapy between the two age groups at baseline with slightly more young individuals using oral estrogen replacement. It is well known that oral estradiol replacement results in more fluctuating plasma estradiol levels compared to those obtained using patches. Therefore, although the baseline estradiol levels were not significantly different between groups, it cannot be excluded that mean 24 h were lower in the young group and may have contributed to higher gonadotropin levels at baseline.
Although both age-groups underwent the same study protocol, and resulting bioavailable estradiol levels were not significantly different between groups during the study, mean estrogen levels were higher at all time points in younger persons. We are unable to explain this unexpected finding; we had no indication that patches were used differently or less meticulously in older subjects. In our analyses, we were unable to adjust for estradiol levels; however, higher mean estrogen levels in older subjects would probably have resulted in lower gonadotropin levels, actually accentuating the observed difference in gonadotropin response between young and older persons.
Also, the gonadotropin levels during the study were more variable than anticipated. We previously executed a similar protocol in healthy male volunteers. The coefficients of variation (CV) for the estradiol levels were similar for the healthy volunteers and the subjects in the present study indicating similar compliance among groups for the study medication. However, CVs for gonadotropin levels were higher in the present study. Although the results of the present study are consistent and straightforward, the statistical power of the study suffered from the higher than expected variability of the gonadotropin levels. Probably, this relates to the increased pulse frequency and/or pulse amplitude in response to a decreased sex hormone feedback signal, however, since we only retrieved single blood samples at any time point we are unable to substantiate this any further.
Additionally, it can be postulated that the difference between unopposed gonadotropin levels in young and older subjects may have been smaller or even non-existent once the unopposed period would have been extended. However, we considered it unethical to leave the participants without hormone substitution for a prolonged period. Therefore, we cannot exclude the possibility that the observed differences between the old and young subjects result form a slower adaptation to the experimental changes in hormonal milieu in the elderly.
In conclusion, the result of our study showed that circulating estradiol has an important inhibitory contribution to LH and FSH release in young and elderly male-to-female transsexuals. Lower gonadotropin levels in older male-to-female transsexuals compared to the young during a low-feedback state supports the hypothesis of a deficient feed-forward drive of the aging hypothalamo-pituitary compartment. The similar suppressive effect of increasing estradiol levels in old and young male-to-female transsexuals argues strongly against our hypothesis of a greater feedback sensitivity of the hypothalamo-pituitary compartment for the feedback effects of estradiol in men.
Acknowledgements
The authors thank Jorn Woerdeman and Jos Megens, who contributed to collect blood samples and helped with recruiting volunteers. This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector. The authors declare that there was no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Zumoff B, Strain GW, Kream J, O'Connor J, Rosenfeld RS, Levin J, Fukushima DK. Age variation of the 24-hour mean plasma concentrations of androgens, estrogens, and gonadotropins in normal adult men. J Clin Endocrinol Metab 1982;54:534–538.
- Davidson JM, Chen JJ, Crapo L, Gray G.D, Greenleaf WJ, Catania JA. Hormonal changes and sexual function in aging men. J Clin Endocrinol Metab 1983;57:71–77.
- Moroz EV, Verkhratsky NS. Hypophyseal–gonadal system during male aging. Arch Gerontol Geriatr 1985;4:13–19.
- Kaufman JM, Vermeulen A. Declining gonadal function in elderly men. Baillieres Clin Endocrinol Metab 1997;11:289–309.
- Morley JE, Kaiser FE, Perry HM, Patrick P, Morley PM, Stauber PM, Vellas B, Baumgartner RN, Garry PJ. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism 1997;46:410–413.
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore longitudinal study of aging. J Clin Endocrinol Metab 2001;86:724–731.
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremmer WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002;87:589–598.
- Baker HW, Burger HG, de Kretser DM, Hudson B, O'Connor S, Wang C, Mirovics A, Court J, Dunlop M, Rennie GC. Changes in the pituitary-testicular system with age. Clin Endocrinol (Oxf) 1976;5:349–372.
- Tsitouras PD, Bulat T. The aging male reproductive system. Endocrinol Metab Clin North Am 1995;24:297–315.
- Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev 2005;26:833–876.
- Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O'Neil TW, Bartfai G, Casanueva F, Forti G, Giwercman A, et al Hypothalamic–pituitary–testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab 2008;93:2737–2745.
- Veldhuis JD. Aging and hormones of the hypothalamo–pituitary axis: gonadotropic axis in men and somatotropic axes in men and women. Ageing Res Rev 2008;7:189–208.
- Longcope C. The effect of human chorionic gonadotropin on plasma steroid levels in young and old men. Steroids 1973;21:583–592.
- Rubens R, Dhont M, Vermeulen A. Further studies on Leydig cell function in old age. J Clin Endocrinol Metab 1974;39:40–45.
- Harman SM, Tsitouras PD. Reproductive hormones in aging men. I. Measurement of sex steroids, basal luteinizing hormone, and Leydig cell response to human chorionic gonadotropin. J Clin Endocrinol Metab 1980;51:35–40.
- Mulligan T, Iranmanesh A, Kerzner R, Demers LW, Veldhuis JD. Two-week pulsatile gonadotropin releasing hormone infusion unmasks dual (hypothalamic and Leydig cell) defects in the healthy aging male gonadotropic axis. Eur J Endocrinol 1999;141:257–266.
- Mulligan T, Iranmanesh A, Veldhuis JD. Pulsatile iv infusion of recombinant human LH in leuprolide-suppressed men unmasks impoverished Leydig-cell secretory responsiveness to midphysiological LH drive in the aging male. J Clin Endocrinol Metab 2001;86:5547–5553.
- Deslypere JP, Kaufman JM, Vermeulen T, Vogelaers D, Vandalem JL, Vermeulen A. Influence of age on pulsatile luteinizing hormone release and responsiveness of the gonadotrophs to sex hormone feedback in men. J Clin Endocrinol Metab 1987;64:68–73.
- Liu PY, Iranmanesh A, Nehra AX, Keenan DM, Veldhuis JD. Mechanisms of hypoandrogenemia in healthy aging men. Endocrinol Metab Clin North Am 2005;34:935–55, ix.
- Mulligan T, Iranmanesh A, Johnson ML, Straume M, Veldhuis JD. Aging alters feed-forward and feedback linkages between LH and testosterone in healthy men. Am J Physiol 1997;273:R1407–R1413.
- Winters SJ, Sherins RJ, Troen P. The gonadotropin-suppressive activity of androgen is increased in elderly men. Metabolism 1984;33:1052–1059.
- Winters SJ, Atkinson L. Serum LH concentrations in hypogonadal men during transdermal testosterone replacement through scrotal skin: further evidence that ageing enhances testosterone negative feedback. The Testoderm Study Group. Clin Endocrinol (Oxf) 1997;47:317–322.
- Winters SJ, Wang C. LH, non-SHBG testostrone and estradiol levels during testosterone replacement of hypogonadal men: further evidence that steroid negative feedback increases as men grow older. J Androl 2009 Dec 3. http://www.andrologyjournal.org/cgi/rapidpdf/jandrol.109.009035v1. Last accessed May 2010.
- Raven G, de Jong FH, Kaufman JM, de Ronde W. In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion. J Clin Endocrinol Metab 2006;91:3324–3328.
- Sherins RJ, Loriaux DL. Studies of the role of sex steroids in the feedback control of FSH concentrations in men. J Clin Endocrinol Metab 1973;36:886–893.
- Marynick SP, Loriaux DL, Sherins RJ, Pita JC Jr, Lipsett MB. Evidence that testosterone can suppress pituitary gonadotropin secretion independently of peripheral aromatization. J Clin Endocrinol Metab 1979;49:396–398.
- Winters SJ, Troen P. Evidence for a role of endogenous estrogen in the hypothalamic control of gonadotropin secretion in men. J Clin Endocrinol Metab 1985;61:842–845.
- Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 1995;80:3689–3698.
- Hayes FJ, Seminara SB, Decruz S, Boepple PA, Crowley WF Jr. Aromatase inhibition in the human male reveals a hypothalamic site of estrogen feedback. J Clin Endocrinol Metab 2000;85:3027–3035.
- Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 β to human plasma proteins at body temperature. J Steroid Biochem 1982;16:801–810.
- Kaufman JM, Giri M, Deslypere JM, Thomas G, Vermeulen A. Influence of age on the responsiveness of the gonadotrophs to luteinizing hormone-releasing hormone in males. J Clin Endocrinol Metab 1991;72:1255–1260.
- Zwart AD, Urban RJ, Odell WD, Veldhuis JD. Contrasts in the gonadotropin-releasing hormone dose-response relationships for luteinizing hormone, follicle-stimulating hormone and α-subunit release in young versus older men: appraisal with high-specificity immunoradiometric assay and deconvolution analysis. Eur J Endocrinol 1996;135:399–406.
- Pincus SM, Mulligan T, Iranmanesh A, Gheorghiu S, Godschalk M, Veldhuis JD. Older males secrete luteinizing hormone and testosterone more irregularly, and jointly more asynchronously, than younger males. Proc Natl Acad Sci USA 1996;93:14100–14105.
- Pincus SM, Veldhuis JD, Mulligan T, Iranmanesh A, Evans WS. Effects of age on the irregularity of LH and FSH serum concentrations in women and men. Am J Physiol 1997;273:E989–E995.
- Stewart-Bentley M, Odell W, Horton R. The feedback control of luteinizing hormone in normal adult men. J Clin Endocrinol Metab 1974;38:545–553.
- Winters SJ, Janick JJ, Loriaux DL, Sherins RJ. Studies on the role of sex steroids in the feedback control of gonadotropin concentrations in men. II. Use of the estrogen antagonist, clomiphene citrate. J Clin Endocrinol Metab 1979;48:222–227.
- T'Sjoen GG., Giagulli VA, Delva H, Crabbe P, De Bacquer D, Kaufman JM. Comparative assessment in young and elderly men of the gonadotropin response to aromatase inhibition. J Clin Endocrinol Metab 2005;90:5717–5722.
- Gooren L. The neuroendocrine response of luteinizing hormone to estrogen administration in the human is not sex specific but dependent on the hormonal environment. J Clin Endocrinol Metab 1986;63:589–593.
- Wortsman J, Hamidinia A, Winters SJ. Hypogonadism following long-term treatment with diethylstilbestrol. Am J Med Sci 1989;297:365–368.