Abstract
Purpose. We performed a randomised controlled study regarding the effects of androgen replacement therapy (ART) on lower urinary tract symptoms (LUTS) in hypogonadal men with benign prostate hypertrophy (BPH).
Methods. Fifty-two patients with hypogonadism and BPH were randomly assigned to receive testosterone (ART group) as 250 mg of testosterone enanthate every 4 weeks or to the untreated control group. We compared International Prostate Symptom Score (IPSS), uroflowmetry data, post-voiding residual volume (PVR) and systemic muscle volume at baseline and 12 months after treatment.
Results. Forty-six patients (ART group, n = 23; control, n = 23) were included in the analysis. At the 12-month visit, IPSS showed a significant decrease compared with baseline in the ART group (15.7 ± 8.7 vs. 12.5 ± 9.5; p < 0.05). No significant changes were observed in the control group. The ART group also showed improvement in maximum flow rate and voided volume (p < 0.05), whereas no significant improvements were observed in the controls. PVR showed no significant changes in either group. In addition, the ART group showed significant enhancement of mean muscle volume (p < 0.05), whereas no significant changes were seen in the controls.
Conclusion. ART improved LUTS in hypogonadal men with mild BPH.
Introduction
Bioavailable testosterone and free testosterone (free T) levels decline with age, and the clinical condition associated with low levels of serum testosterone (T) with specific symptoms is called late-onset hypogonadism (LOH) syndrome. The most widely accepted parameter used to establish a diagnosis of LOH syndrome is the measurement of serum total T. In Japan, LOH is diagnosed based on the serum free T level. The Massachusetts Male Aging Study gave crude prevalence estimates for hypogonadism in men ranging from 6.0% to 12.3% between age 40 and 69 years, and estimated that 2.4 million men in the USA have androgen deficiency [Citation1]. The most widely recognised clinical signs of LOH syndrome are decreases in muscle mass and strength, a decrease in bone mineral density, decreases in libido and sexual desire and an increase in visceral fat [Citation2,Citation3], which may have significant effects on quality of life and adversely affect the function of multiple organ systems [Citation4–7]. Furthermore, it has recently been noted that LOH syndrome itself is an important sign of many potentially serious conditions, which can be avoided or treated by androgen replacement therapy (ART). ART improves many of the LOH-associated symptoms and conditions, and its clinical use has increased substantially over the past several years. On the other hand, ART may be associated with worsening of prostate cancer, liver toxicity, worsening of sleep apnea syndrome, erythrocytosis and gynecomastia [Citation2,Citation3].
Benign prostate hypertrophy (BPH) is one of the most common diseases in older men. The presence of androgen is required for the development of BPH, and antiandrogen agents can decrease prostate volume in patients with BPH [Citation8], suggesting that ART may result in worsening of BPH symptoms. However, there are no compelling data suggesting that ART contributes to worsening of lower urinary tract symptoms (LUTS) or promotion of urinary retention [Citation2]. Here, we performed a randomised controlled study to investigate the effects of ART on LUTS in hypogonadal men with BPH.
Methods
Screening of participants
Patients with BPH at Kanazawa University Hospital were screened for this study by measuring serum free T level. BPH had been diagnosed based on the criteria described previously in Japan; International Prostate Symptom Score (IPSS) >7 and total prostate volume >20 ml [Citation9]. The biochemical diagnosis of hypogonadism was made based on Japanese biochemical criteria as follows: free T ≤ 8.5 pg/ml, ART is the first choice of treatment for hypogonadism; free T 8.5–11.8 pg/ml, ART is a relative choice [Citation10], and the patients with free T value of <11.8 pg/ml were eligible in this study. Patients with serum creatinine (Cr) or alanine aminotransferase (ALT) levels more than 1.5 times the upper limits of the normal range at the screening visit were excluded from the study. Furthermore, patients with prostate specific antigen (PSA) level >2.0 ng/ml, prostate cancer, or administration of antiandrogen agents within 6 months were also excluded from the study. The study protocol was approved by Kanazawa University Hospital Institutional Review Board. All subjects gave informed written consent before both screening tests for recruitment and participation in the study. Ninety-seven men with BPH received the screening test, and 52 patients with a diagnosis of hypogonadism were enrolled in the study.
Protocol
At the screening visit, blood data, free T, PSA, AST, and Cr, were determined. Moreover, a complete medical history was taken and physical examination was performed. After obtaining informed consent for participation in the study, each eligible subject underwent transurethral ultrasonography (TRUS) to determine prostate volume, and uroflowmetry (UFM) analysis, post-voiding residual (PVR) volume measurement, aging males symptoms (AMS) score and IPSS questionnaire survey were performed. PVR was evaluated by ultrasonographic examination. In addition, muscle mass volume was analysed using aBody Planner™ DF800 (Yamato Biospace Technology, Hyogo Japan), which can be used to evaluate systemic body muscle in eight phases (0–7), to confirm the effects of ART.
After completion of all baselines tests, participants were randomly assigned to receive testosterone administration (ART group) and to the untreated control group. In the ART group, 250 mg of testosterone enanthate (Enarmone Depot™; ASKA Pharmaceutical Co., Ltd., Tokyo, Japan) was injected intramuscularly every 4 weeks. Safety evaluation was performed in each subject, including physical examination and blood testing at 3, 6 and 12 months after the baseline visit, and PSA was measured at baseline, 6-, and 12-month visits. AMS score, IPSS questionnaire, UFM, PVR and muscle volume measurement were evaluated at baseline and12-month visit.
During the period of this trial, in principle, we did not change any drugs associated with urinary symptoms in patients receiving medication. Patients who required additional medication or changes of drugs because of worsening of their urinary symptoms were excluded from analysis.
Statistical analysis
Comparison of all baseline clinical variables between the ART and control groups was performed by the Mann–Whitney rank sum test or χ2 test. For each group, the changes in each parameter were compared by Wilcoxon's signed rank test, and changes from baseline in the two groups were evaluated using Student's t test. Frequencies of side effects were compared using the χ2 test. All statistical analyses were performed using SPSS™ version 17.0 Medical Model (SPSS Inc., Chicago, IL). Statistical significance was defined as p < 0.05.
Results
Clinical characteristics
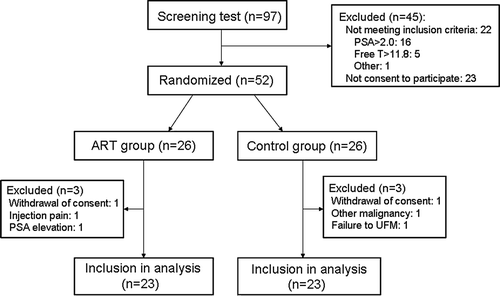
A total of 97 patients with BPH underwent screening examination, and 22 patients who did not meet the inclusion criteria were excluded from the study (). Moreover, 23 patients who did not consent to participation in the study were also excluded. A total of 52 patients (ART group, n = 26; control, n = 26) were randomized into two groups, and 6 patients who did not complete this trial were excluded (). Finally, 46 patients (ART group, n = 23; control, n = 23) were included in the analysis. The clinical characteristics of the subjects are shown in , which compares baseline clinical characteristics in patients randomised to the ART group with those randomised to the control group. There were no significant differences between the two groups in any of the parameters examined. In the present study, severity of BPH was mild, with mean prostatic adenoma volumes of 11.0 ± 13.7 and 8.8 ± 6.0 ml in the ART and control groups, respectively.
Table I. Comparison of patients background.
Urinary function based on IPSS, UFM and PVR
At the 12-month visit, IPSS showed a significant decrease compared with baseline from 15.7 ± 8.7 to 12.5 ± 9.5 (p < 0.05) in the ART group, and a slight decrease from 14.0 ± 10.1 to 13.5 ± 9.8 in the control group (p = 0.345) ( ). In the sub-scores of IPSS, the scores for question 2 (Q2: ‘Over the past month, how often have you had to urinate again less than two hours after you finished urinating?’) and question 5 (Q5: ‘Over the past month, how often have you had a weak urinary stream?’) were significantly improved after 12 months of ART (p < 0.05) (). There were no significant changes in any of the sub-scores of IPSS over this period in the control group.
Figure 2. Changes in IPSS sub-scores from baseline to 12-month visit in ART group (A) and control group (B) were shown. IPSS sub-scores showed significant improvements in Q2 and Q5 among ART group (p < 0.05). The details of IPSS questionnaires were shown as following; Q1: how often have you had a sensation of not emptying your bladder completely after you finished urinating? Q2: how often have you had to urinate again less than two hours after you finished urinating? Q3: how often have you found you stopped and started again several times when you urinated? Q4: how often have you found it difficult to postpone urination? Q5: how often have you had a weak urinary stream? Q6: how often have you had a push or strain to begin urination? Q7: how many times did you most typically get up to urinate from the time you went to bed at night until the time you got up in the morning?
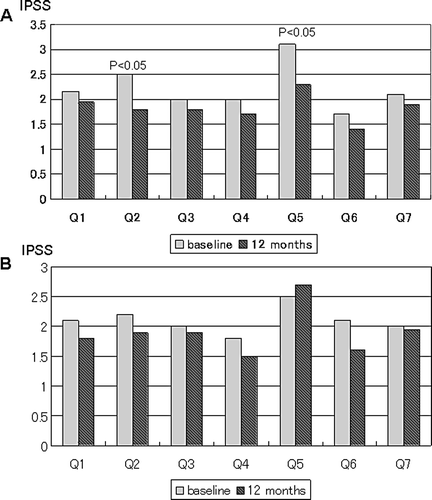
Table II. Comparison of parameters at baseline and at 12-month visit in the two groups.
Based on analysis by UFM, the patients receiving ART reported significant improvement in maximum flow rate (MFR) (from 12.9 ± 5.6 to 16.7 ± 9.5 ml/s, p < 0.05) and a significant increase in voided volume (VV) (from 253 ± 120 to 283 ± 145 ml/s, p < 0.05) at the end of ART, whereas the control group did not show significant changes in MFR or VV. On the other hand, PVR showed no significant changes in either the ART group or the controls.
Comparison of changes from baseline to 12-month visit in the two groups also showed that ART could improve IPSS, MFR, and VV and have little effect on PVR in patients with mild BPH and hypogonadism ().
Table III. Comparison of changes from baseline to 12-month visit between the two groups.
AMS score and muscle mass volume
AMS score showed no significant changes in either the ART group or the controls over this trial period (). We also evaluated changes of systemic muscle volume to confirm the effects of ART. The patients who received ART tended to show greater mean muscle volume at the end of the trial (from 3.22 ± 1.83 to 3.83 ± 2.00; p < 0.05), whereas there were no significant changes in the control group compared with baseline ().
Comparison of changes from baseline to 12-month visit in two groups also showed that ART could enhance systematic body muscle, demonstrating the effectiveness of testosterone administration in the ART group ().
Safety
One patient in the ART group discontinued treatment because of sudden PSA elevation from 1.190 ng/ml at baseline to 8.719 ng/ml at the 6-month visit (). Chronic prostatitis was suspected based on the findings of transrectal ultrasonography and digital rectal examination, and PSA value showed an immediate decline (1.350 ng/ml) one month after ART interruption. Thus, prostate biopsy was not performed in this case. Mean PSA values showed a small but significant increase in both the ART group and control group (), but there was no significant difference in the change of PSA value from baseline to 12 months after the trial between the groups (). There were no patients who required prostate biopsy or had a diagnosis of prostate cancer in this period. There were no other severe adverse events that resulted in withdrawal from the trial. Urinary complications, such as severe exacerbation of voiding symptoms or urinary retention, did not occur in either group during the trial period. Furthermore, no patients required additional medication or changed drugs because of worsening of their urinary symptoms during the period of this trial.
Discussion
Concurrent with the progressive decline in testosterone from middle age, there is a gradual increase in prostate volume, reflecting the evolution of BPH, a common disease of later life affecting older men [Citation11]. The prostate is well known as a clinical androgen-dependent organ, and marked decreases in prostate volume have been observed associated with orchidectomy and administration of antiandrogen drugs [Citation12]. Generally, it is not recommended to prescribe testosterone for patients with prostate cancer [Citation13], and every effort should be made to detect or exclude the presence of prostate cancer before ART is initiated. Moreover, the PSA value must be monitored during ART to detect any possible incidence of prostate cancer at an early stage.
The relationships between ART and the risk of progression of BPH or urinary symptoms have also been discussed in the literature. A short-term clinical study using 100 mg of testosterone enanthate every 3 weeks for 3 months did not significantly increase prostate volume or PVR [Citation14]. Another double-blind controlled study indicated that ART contributed to an increase of 12% in volume of the prostate gland after 8 months of treatment, but did not affect UFM data, PVR or IPSS [Citation15]. In an 11-month study, ART resulted in no subjective increase in prostate size or deterioration of voiding symptoms [Citation16]. Moreover, a long-term clinical study using oral testosterone undecanoate for 10 years in 33 men demonstrated a slight decrease in urine flow but no increase in prostate size and no evidence of cancer development [Citation17]. Another interesting double-blind placebo-controlled study indicated that 6 months of testosterone administration normalised serum androgen levels in aging men with hypogonadism but had little effect on prostatic tissue androgen levels, such as those of testosterone and DHT, or on their urinary symptoms and UFM data [Citation18]. Many other studies failed to show a significant worsening of MFR, PVR, and voiding symptoms caused by BPH during ART, and complications, such as urinary retention, did not occur at higher rates than in controls [Citation19,Citation20]. These studies demonstrated that ART has little negative effect on urinary function or prostate volume.
In the present study, we demonstrated that ART for hypogonadal men with mild BPH could contribute to improvement of LUTS, MFR and VV. To our knowledge, this is the first randomised controlled study to demonstrate that ART may also be beneficial for urinary symptoms in patients with mild BPH. An uncontrolled study indicated progressive reduction of prostate volume and improvement of urinary symptoms among 207 middle-aged and older men who were treated with oral testosterone undecanoate for 6 months [Citation19]. This previous report indicated that ART resulted in a decrease of approximately 30% in prostate volume and a significant decrease of IPSS score. On the other hand, they also reported that 20 patients without suppression of plasma LH reflecting defects in hypothalamic–pituitary regulation in testicular function, suggesting failure to demonstrate a consistent increase in plasma testosterone level, did not show a decrease in prostate volume. These findings suggest that successful ART could improve urinary symptoms and BPH directly.
We found significant increases in MFR and VV in the ART group based on the results of UFM analysis. Furthermore, IPSS sub-scores showed improvements in categories associated with MFR (Q5) and VV (Q2). There are several possible explanations for the improvement of LUTS and bladder function by testosterone therapy. An uncontrolled prospective study indicated that testosterone replacement with transdermal testosterone 50–100 mg gel per day for 1 year in 25 men with LOH syndrome significantly increased maximal bladder capacity and compliance, and decreased detrusor pressure at maximal flow, based on the results of pressure-flow analysis [Citation21]. An experimental study of male rabbits subjected to bilateral orchiectomy showed that testosterone injection significantly increased bladder capacity and compliance with high blood testosterone levels [Citation22]. Madeiro et al. reported that the bladders of castrated rats receiving androgen therapy for 28 days showed a greater number of vessels, epithelial thickness and quantity of muscular fibres than untreated controls [Citation23]. These previous findings suggest that ART can enhance bladder muscle contractility and compliance, which may lead to increases in MFR and VV.
Androgen deprivation can inhibit the smooth muscle differentiation pathway. Androgen stimulates stromal precursor cells to differentiate into smooth muscle cells [Citation24]. Cayan et al. reported that testosterone treatment resulted in significantly higher bladder smooth muscle/collagen ratio than controls in rats [Citation25]. The improvement of LUTS by ART may be associated with such pathological changes in the bladder smooth muscle, and enhanced smooth muscle may lead to increase of bladder contraction and compliance. However, in the present study, pathological changes in the bladder associated with ART were not evaluated to avoid invasive examination. Thus, further studies using combinations of urodynamic analysis and histological analysis of the bladder are required to confirm this hypothesis.
As an alternative explanation, testosterone administration has been shown to have an effect on Rho-kinase activity, which is associated with calcium sensitivity of the contractile machinery in bladder smooth muscle or the prostate in experimental animal models with bladder outlet obstruction [Citation26]. In addition, a decrease in bladder blood flow has been suggested in patients with BPH [Citation27], and decreased bladder blood flow and ischemia caused by aging and arterial sclerosis is associated with the development of detrusor overactivity and LUTS [Citation28,Citation29]. Furthermore, chronic bladder ischemia is considered attributable to bladder distention induced by bladder outlet obstruction [Citation30]. Testosterone activates endothelial nitric oxide synthase, and consequently alleviates pelvic ischemia. For several reasons, ART may improve bladder function in hypogonadal patients with mild BPH.
The limitations of the present study include the small sample size and the lack of data based on urodynamic study. Moreover, the severity of BPH in the target population was mild, and the present results may not necessarily be applicable to patients with severe BPH. Therefore, further studies are required to allow us to draw more definitive conclusions regarding the associations between testosterone therapy and urinary function. Furthermore, PSA values showed small but significant increases associated with ART for 12 months. However, there was no significant difference in PSA from baseline to the end of trial between the two groups. Long-term follow-up regarding safety of ART is also necessary.
In conclusion, the present randomised controlled study suggested that ART may improve LUTS in hypogonadal men with mild BPH by increasing MFRand VV, in addition to increasing muscle mass volume.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Araujo AB, O'Donnell AB, Brambilla DJ, Simpson WB, Longcope C, Matsumoto AM, McKinlay JB. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 2004;89:5920–5926.
- Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag 2009;5:427–448.
- Comhaire FH. Andropause: hormone replacement therapy in the ageing male. Eur Urol 2000;38:655–662.
- Morales A, Lunenfeld B. Standards, guidelines and recommendations of the international society for the study of the aging male (ISSAM). Investigation, treatment and monitoring of late-onset hypogonadism in males. Official recommendations of ISSAM. Aging Male 2002;5:74–86.
- Lunenfeld B, Saad F, Hoesl CE. ISA, ISSAM and EAU recommendations for the investigation, treatment and monitoring of late-onset hypogonadism in males: scientific background and rationale. Aging Male 2005;8:59–74.
- Nieschlag E, Swerdloff R, Behre HM, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morley JE, Schulman C, Wang C, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male 2005;8:56–58.
- Wang C, Nieschlag E, Swerdloff RS, Behre H, Hellstrom WJ, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morales A, et al. ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male 2009;12:5–12.
- Roehrborn CG, Siami P, Barkin J, Damião R, Major-Walker K, Nandy I, Morrill BB, Gagnier RP, Montorsi F; CombAT Study Group. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT Study. Eur Urol 2010;57:123–131.
- Homma Y, Kawabe K, Tsukamoto T, Yamaguchi O, Okada K, Aso Y, Watanabe H, Okajima E, Kumazawa J, Yamaguchi T, et al. Estimate criteria for diagnosis and severity in benign prostatic hyperplasia. Int J Urol 1996;3:261–266.
- Namiki M, Akaza H, Shimazui T, Ito N, Iwamoto T, Baba K, Kumano H, Koh E, Tsujimura A, Matsumiya K, et al. Clinical practice manual for late-onset hypogonadism syndrome. Int J Urol 2008;15:377–388.
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol 1984;132:474–479.
- Huggins C, Stevens RE Jr, Hodges CV. Studies on prostatic cancer. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg 1941;43:209–223.
- Prehn RT. On the prevention and therapy of prostate cancer by androgen administration. Cancer Res 1999;59:4161–4164.
- Tenover JS. Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab 1992;75:1092–1098.
- Holmäng S, Mårin P, Lindstedt G, Hedelin H. Effect of long-term oral testosterone undecanoate treatment on prostate volume and serum prostate-specific antigen concentration in eugonadal middle-aged men. Prostate 1993;23:99–106.
- Franchi F, Luisi M, Kicovic PM. Long term study of testosterone undecanoate in hypogonadal males. Int J Androl 1978;1:270–278.
- Gooren LJ. A ten-year safety study of the oral androgen testosterone undecanoate. J Androl 1994;15:212–215.
- Marks LS, Mazer NA, Mostaghel E, Hess DL, Dorey FJ, Epstein JI, Veltri RW, Makarov DV, Partin AW, Bostwick DG, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA 2006;296:2351–2361.
- Pechersky AV, Mazurov VI, Semiglazov VF, Karpischenko AI, Mikhailichenko VV, Udintsev AV. Androgen administration in middle-aged and ageing men: effects of oral testosterone undecanoate on dihydrotestosterone, oestradiol and prostate volume. Int J Androl 2002;25:119–125.
- Slater S, Oliver RT. Testosterone: its role in development of prostate cancer and potential risk from use as hormone replacement therapy. Drugs Aging 2000;17:431–439.
- Karazindiyanoğlu S, Cayan S. The effect of testosterone therapy on lower urinary tract symptoms/bladder and sexual functions in men with symptomatic late-onset hypogonadism. Aging Male 2008;11:146–149.
- Celayir S. Effects of different sex hormones on male rabbit urodynamics: an experimental study. Horm Res 2003;60:215–220.
- Madeiro A, Girão M, Sartori M, Acquaroli R, Baracat E, Rodrigues De Lima G. Effects of the association of androgen/estrogen on the bladder and urethra of castrated rats. Clin Exp Obstet Gynecol 2002;29:117–120.
- Traish AM, Goldstein I, Kim NN. Testosterone and erectile function: from basic research to a new clinical paradigm for managing men with androgen insufficiency and erectile dysfunction. Eur Urol 2007;52:54–70.
- Cayan F, Tek M, Balli E, Oztuna S, Karazindiyanoğlu S, Cayan S. The effect of testosterone alone and testosterone + estradiol therapy on bladder functions and smooth muscle/collagen content in surgically menopause induced rats. Maturitas 2008;60:248–252.
- McVary K. Lower urinary tract symptoms and sexual dysfunction: epidemiology and pathophysiology. BJU Int 2006;97 (Suppl 2):23–28.
- Mitterberger M, Pallwein L, Gradl J, Frauscher F, Neuwirt H, Leunhartsberger N, Strasser H, Bartsch G, Pinggera GM. Persistent detrusor overactivity after transurethral resection of the prostate is associated with reduced perfusion of the urinary bladder. BJU Int 2007;99:831–835.
- McVary KT. Erectile dysfunction and lower urinary tract symptoms secondary to BPH. Eur Urol 2005;47:838–845.
- Azadzoi KM, Tarcan T, Kozlowski R, Krane RJ, Siroky MB. Overactivity and structural changes in the chronically ischemic bladder. J Urol 1999;162:1768–1778.
- Okutsu H, Matsumoto S, Hanai T, Noguchi Y, Fujiyasu N, Ohtake A, Suzuki M, Sato S, Sasamata M, Uemura H, et al. Effects of tamsulosin on bladder blood flow and bladder function in rats with bladder outlet obstruction. Urology 2010;75:235–240.