Abstract
This article reviews most common types of thyroid cancer focusing on older males worldwide. Thyroid carcinoma is the most common endocrine malignancy. It includes tumour types that range from localised papillary carcinomas to lethal anaplastic disease. Among elderly population, male gender seems to be highly correlated with the risk and aggressiveness of thyroid cancer. Early diagnosis and urgent aggressive treatment are important for aging patients. This article includes numerous studies which evaluate prevalence, morbidity and mortality of thyroid cancer in older males.
Introduction
The medical literature describes cancer being the most common cause of death in the population over 60 years of age. Although cardiovascular disease is still considered to be the leading cause of death worldwide, the steadily increasing number of elderly patients with cancer is becoming a health concern for industrialised countries [Citation1,Citation2].
While thyroid cancer is one of the least common cancers in older men, studies have reported an increasing incidence of this particular cancer since 1980s [Citation3,Citation4]. Aging represents an important factor to define the aggressiveness of thyroid carcinomas. Among elderly population, male sex seems to be highly correlated with the risk of thyroid cancer. Even though data from the aging population confirms that men are less likely to be affected by thyroid disease than women, male gender may represent a risk factor for thyroid cancer. This observation should be carefully considered in the evaluation of thyroid nodules in the elderly [Citation5].
Diagnosis
The prevalence of thyroid nodules increases with age. While goitres and multinodular glands are known to be more common in elderly, 6–10% of older population has solitary nodules by palpation. Nearly 50% of population has nodules detectable by ultrasonography by the age of 65 [Citation6]. Thyroid nodules can represent cysts, benign adenomas, inflammation or cancer.
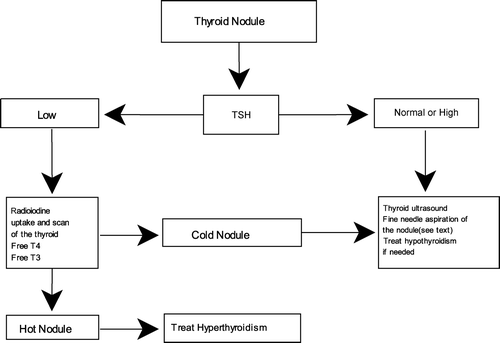
The approach to a solitary nodule in an older person is the same as in a younger patient. Serum Thyroid-Stimulating Hormone (TSH) measurement may be used as a first-line screening test. An undetectable TSH suggests toxic nodule and should lead to obtaining radioiodine scan and uptake. However, patients with thyroid cancer rarely have abnormalities in serum TSH levels [Citation7]. If the thyroid tests are normal, fine-needle aspiration (FNA) of the nodule should be performed for further evaluation. Most studies recommend investigating nodules of more than 1 cm in diameter and those with clinical or sonographic suspicious findings [Citation8,Citation9]. Diagnosis of thyroid nodule is summarized in .
Nodules occurring at the extremes of age, particularly in men, are especially likely to be cancerous. A retrospective review of 3629 patients revealed two peaks in age for thyroid cancer – one in patients aged 20–29 years and second in patients over 65 years of age [Citation10]. One study revealed the odds of cold nodule being cancer in men quadrupled by the age of 64 and reached a frequency of more than 50% by 70 years of age [Citation11]. Other risk factors of a thyroid nodule being malignant include neck irradiation during childhood or adolescence, rapid growth, recent changes in speaking, breathing or swallowing, family history of thyroid malignancy or multiple endocrine neoplasia type 2, firm and irregular consistency of nodule, vocal cord paralysis, regional lymphadenopathy, ultrasound findings of hypoechoic lesions, irregular margins, presence of calcifications without shadowing, absence of halo and internal or central blood flow [Citation7,Citation12]. Approximately 5% of palpable nodules are diagnosed as malignant [Citation13].
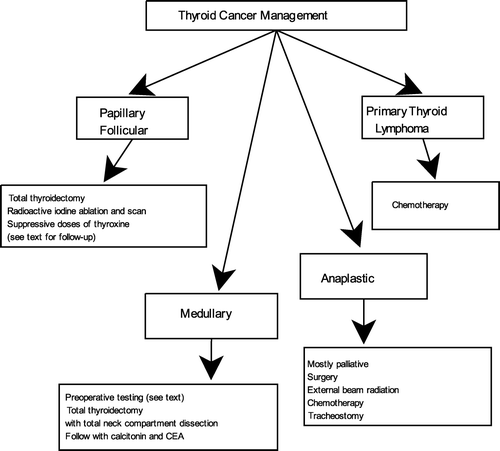
The four main types of thyroid cancer include papillary, follicular, anaplastic and medullary thyroid cancer. Primary thyroid lymphoma is a relatively rare thyroid malignancy. Key clinical features of thyroid cancer types are summarized in . Both follicular and anaplastic histotypes of thyroid cancer are more frequently found in older patients compared to younger population. A retrospective analysis of 204 thyroid cancer patients aged 60 years or older revealed 70% of thyroid cancers were well-differentiated (68% papillary, 30% follicular and 2% hurthle), 2% were medullary thyroid cancer and 29% were non-well-differentiated (19% anaplastic, 4% metastatic cancers to thyroid, 3% lymphoma and 2% squamous cell) [Citation14]. For well-differentiated thyroid carcinomas, male gender, follicular carcinoma and a larger tumour size indicated a poor prognosis.
Table I. Types and key features of thyroid cancer.
Papillary thyroid cancer
Papillary thyroid cancer is considered to be the most common type of thyroid cancer, with the median age of diagnosis being close to 45 years of age. Overall, thyroid cancer is diagnosed in women two to three times more often than in men [Citation15]. However, looking at individual age categories changes this observation. One epidemiology study examining gender and age-specific effect for papillary thyroid cancer incidence showed a declining female-to-male ratio with age. Although, age-specific rates were higher among women than men across all age groups, the female-to-male rate ratio decreased quite consistently from 3.4 at ages 35–44 to 1 at ages 80+ [Citation16].
Most patients present with asymptomatic thyroid mass found by the patient or their healthcare provider. As previously discussed, thyroid function tests are typically normal. Papillary thyroid cancer spreads via lymphatic system to the regional lymph nodes. Rare cases of metastasis involve the lungs, bones, brain and soft tissues. A few studies reveal that 80–90% of patients have microscopic lymph node involvement at the time of diagnosis with meticulous neck dissection and careful pathologic examination [Citation17,Citation18]. Distant metastatic spread at diagnosis is seen in only 2–5% of cases [Citation19]. Despite that, papillary cancer is generally associated with low mortality in patients younger than 40 years old.
The mortality rate is usually higher in older population. A retrospective study done in Spain, looking at survival of 200 papillary cancer patients, found a poorer prognosis for patients of 50 years of age and older [Citation20]. According to Mazaferri [Citation21] and Godballe et al. [Citation22], an increased mortality in papillary cancer patients occurs from 60 years onward. Tumours from patients older than 60 years of age show significantly more mitotic activity and nuclear polymorphism, fewer psammoma bodies and more frequent extrathyroidal invasion and distant metastases [Citation22]. These results indicate that 60 years of age at the time of diagnosis may be the prognostic break-point for papillary thyroid carcinoma. Another retrospective study looked at 10-year survival rates in age-specific population. Patients who were younger than 40 years of age had 100% survival rate. Papillary thyroid cancer patients, who were 41–60 years old, had 92% survival rate. Survival rate decreased to 76% in patients older than 60. While 10-year survival rate in all males was 85% versus 94% in all females, this difference was not statistically significant [Citation23].
Other studies reveal some gender-related differences [Citation24–26]. The tall cell variant of papillary thyroid cancer, reported in 1976, is found to be present in large tumours, usually over 5 cm in diameter in older male patients, and is associated with extra thyroidal manifestations and increased risk for cancer-related death [Citation24]. Machens et al. followed 587 papillary thyroid cancer patients. Primary diameters of sporadic tumours were significantly (p < 0.001) larger in male patients [Citation25]. Review of 257 Swedish subjects with papillary thyroid cancer confirmed age of more than 65 and male gender to be the unfavourable influence on survival. Ten-year relative survival rates for cases diagnosed in 1988–1993 were 94% for papillary cancer in females and 69% in males [Citation26]. Another study found the recurrences of papillary thyroid cancer were at the extremes of age (<20 and >59). The likelihood of cancer recurrence and death were reduced by female gender among other factors [Citation27].
Follicular thyroid cancer
Follicular thyroid cancer is the second most common tumour of the thyroid gland. It accounts for up to 15% of all cases of thyroid cancer areas where diets are supplemented with iodine, and 25–40% of cases in iodine-deficient areas [Citation28,Citation29]. Follicular thyroid cancer usually occurs in older people, with the mean age in most studies being more than 50 years, about 10 years older than that for typical papillary thyroid cancer [Citation30]. Follicular carcinoma has cytologic features similar to follicular adenoma, but is distinguished from the benign lesion on the basis of vascular invasion, capsular invasion, adjacent thyroid tissue invasion, lymph node metastasis or systemic metastases [Citation31]. Hurthle cell neoplasm requires similar criteria for malignancy. These tumours are more likely to invade blood vessels and metastasize hematogenously to distant sites, most commonly bone and lung, compared to papillary carcinoma [Citation32].
Radiation exposure to the thyroid gland is known to increase the incidence of follicular and hurthle cell neoplasia. Patient population, who underwent childhood irradiation for benign conditions like thymic enlargement and acne between 1920 and 1960, is now between the ages of 49 and 89. This group of patients still has an increased risk of developing thyroid carcinoma, since there is no apparent decrease in the elevated risk even 40 years after the radiation exposure [Citation33].
Similar to papillary thyroid cancer, follicular thyroid neoplasia is more common in females than males. It may present as an asymptomatic neck mass, usually without palpable cervical lymph nodes [Citation34]. The larger the thyroid nodule, the more chance it has to be malignant. Although follicular thyroid cancer is more common in females, large thyroid nodules found in males are more likely to be malignant as was shown by Tuttle et al. The study shows that the risk of malignancy is significantly higher when follicular neoplasia is present in a male (43% vs. 16% for females, p = 0.007), when the nodule is greater than 4 cm to palpation, or when the nodule is judged to be solitary by palpation. The risk of malignancy in males with large nodules was nearly 80% after an FNA showing a follicular neoplasm [Citation35]. Since follicular thyroid cancer has tendency to metastasize, it is associated with a higher mortality than papillary thyroid cancer. Studies are reporting age as one of the independent factors for worse prognosis and likelihood of metastatic disease. Crile et al. reported a worse prognosis for follicular thyroid cancer patients older than 60 years of age [Citation36].
Medullary thyroid cancer
Medullary thyroid cancer derives from the neuroendocrine C cells of the thyroid. It represents 2–5% of all thyroid malignancies. Unlike papillary and follicular thyroid cancers, most of which are sporadic in nature, medullary thyroid carcinoma occurs sporadically in about 80% of patients, and about 20% of patients have a hereditary variety [Citation37]. Hereditary form of medullary carcinoma is more common in younger population, while the sporadic form is more common in older patients with mean age at presentation of about 47 years [Citation38]. The hereditary variety can be transmitted as a familial medullary thyroid carcinoma, or it can arise as part of multiple endocrine neoplasia syndrome type 2A or 2B [Citation37]. All familial forms of the disease are inherited in autosomal dominant fashion, with a germline mutation in the tyrosine kinase proto-oncogene RET (REarranged during Transfection) [Citation39]. Hereditary form is typically bilateral [Citation38]. Metastatic cervical adenopathy is noted in about 50% of patients at initial presentation.
The ability of the parafollicular cells to oversecrete calcitonin and sometimes other hormonally active peptides may lead to unexplained diarrhoea, facial flushing and symptoms of Cushing's syndrome in patients with advanced disease. The diagnosis of medullary thyroid carcinoma can be established after a FNA aspiration biopsy. Positive immunocytochemical staining for calcitonin allows confirmation of the diagnosis [Citation40]. Although some experts suggest measuring serum calcitonin routinely for all thyroid nodule evaluations, it is not cost-effective and results can be misleading [Citation41,Citation42].
Patients with inherited disease seem to have a better outlook compared to sporadic disease [Citation39]. Overall, patients under 40 years of age at diagnosis have a 5-year and 10-year survival of around 95% and 75%, respectively, which drops to 65% and 50% for those older than 40 years [Citation43]. Kebebew et al. followed 104 medullary thyroid cancer patients. Patients younger than 45 years of age, female gender and with medullary thyroid cancer confined to the thyroid had the best overall prognosis – 100% survival at 10 years [Citation38]. Another study done by Machens et al. looked at 320 patients with sporadic medullary thyroid cancer patients and 159 patients with hereditary medullary thyroid cancer. Sporadic medullary thyroid cancers were significantly larger size in male patients. In addition, extrathyroidal extension of sporadic medullary cancers (35% vs. 15%, p < 0.001) and distant metastasis from sporadic medullary cancers at the most recent operation (29% vs. 15%, p = 0.002) were seen significantly more often with male patients. No significant gender differences were observed in patients with hereditary medullary cancers [Citation25]. A retrospective study of 109 patients with sporadic medullary thyroid carcinoma revealed male sex and age > 60 years, among other characteristics, to be risk factors for unfavourable outcome [Citation44].
Anaplastic thyroid cancer
Anaplastic thyroid cancer is a rare, usually lethal malignancy of older adults with no effective systemic therapy. According to the National Cancer database of thyroid carcinomas registered in the United States between 1985 and 1995, 2% were anaplastic thyroid carcinoma [Citation45]. This type of thyroid cancer typically presents in the seventh decade of life, with more than 90% of patients older than 50 years [Citation46,Citation47]. Women make up around 60% of affected population [Citation48]. Anaplastic thyroid cancer usually presents as a rapidly growing hard neck mass, with a mean tumour size of 6–9 cm. Most patients have widespread local invasion, vocal cord paralysis and high frequency of distant metastases in bone, lung, pleura and brain [Citation49]. The most common presenting signs and symptoms also include hoarseness, dysphagia, neck pain, weight loss, dyspnea, stridor and cough [Citation50]. Some cases present as the sudden rapid growth of a longstanding goitre over the previous few weeks or months [Citation51]. The diagnosis of the disease is usually established by FNA aspiration or surgical biopsy. Neck and mediastinum CT can help to determine the extent of the thyroid tumour and to identify tumour invasion of great vessels and other vital structures [Citation39].
Age at diagnosis of anaplastic thyroid carcinoma is considered to be a strong predictor of prognosis. Kim et al. looked at 121 subjects with anaplastic thyroid carcinoma. Age less than 60 years, tumour size less than 7 cm and lesser extent of disease were independent predictors of lower cause-specific mortality on multivariate analysis [Citation52]. Pierie et al. observed that age less or equal to 70 years was an independent predictor of survival [Citation53]. The cohort, consisting of 516 patients with anaplastic thyroid cancer registered between 1973 and 2000, showed that age less than 60 years, female gender, intrathyroidal tumour, external beam radiotherapy, surgical resection and combined surgical resection of tumour and radiotherapy were associated with a lower cause-specific mortality by univariate analysis. On multivariate analysis, survival gender differences were not statistically significant [Citation54]. Tan et al. reported tumour size less than 6 cm and female gender were significant prognostic factors [Citation55]. Gilliland et al. looked at 251 anaplastic thyroid cancer patients. The absolute differences between relative survival for males and females were small, indicating that gender was not a strong predictor of survival [Citation48]. The mean survival time is frequently less than 6 months from diagnosis [Citation46]. Death is secondary to upper airway obstruction and suffocation in 50% of patients as well as combination of local and distant disease [Citation56].
In summary, anaplastic thyroid cancer is a rapidly growing tumour, which is more common in older patients. While age appears to be a strong predictor of prognosis, it is not the same case for gender differences.
Primary thyroid lymphoma
Primary thyroid lymphoma is another rare type of thyroid cancer, which accounts for 1–5% of all thyroid malignancies and about 1–2% of all extranodal lymphomas with annual incidence of two per million [Citation57]. It is more common in women with a 3 to 1 predominance and has a peak incidence in the late 60s. Patients present with a rapidly growing neck mass, hoarseness, dysphagia or stridor [Citation58,Citation59]. Primary thyroid lymphoma can be classified by histologic subtype. The most common include large B-cell lymphoma, marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue and follicular lymphoma [Citation60]. Patients with Hashimoto's thyroiditis are at increased risk of developing primary thyroid lymphoma. There is especially strong association between Hashimoto's thyroiditis and mucosa-associated lymphoid tissue lymphoma [Citation61].
It is may be difficult to make a diagnosis by FNA aspiration biopsy alone due to the histopathological similarities between primary thyroid lymphoma and Hashimoto's disease. In a recent analysis, FNA aspiration biopsy accurately diagnosed primary thyroid lymphoma in only 60% of patients who were later proven to have the malignancy [Citation62]. Combination of FNA aspiration biopsy and flow cytometry of FNA aspiration material may accurately diagnose primary thyroid lymphoma. In addition, patients may require excisional or open surgical biopsy for diagnosis confirmation [Citation60]. Ultrasonography can be used to document precise measurements, confirm an increase in size of a nodule or goitre, and help to obtain a representative tissue sample on biopsy.
Older age at diagnosis is associated with decreased survival. Graff-Baker et al. gathered data on 1408 cases of primary thyroid lymphoma. Age > or = 80 years, advanced stage, no radiation or surgery, and large B-cell or follicular histology predicted worse prognosis in multivariate analysis. There appeared to be no sex differences in prognosis [Citation63]. A retrospective analysis of prognostic factors showed mediastinal lymph node involvement and performance status to be the most important prognostic factors in primary lymphoma patients. Gender among other factors did not seem to influence survival [Citation59].
Treatment
Management of the thyroid cancer depends on the type of malignancy as summarized in . Surgical treatment with total thyroidectomy is favoured in most cases of papillary and follicular thyroid cancers. Total thyroidectomy decreases the recurrence rate because many papillary thyroid cancers are multifocal and bilateral. It also facilitates successful remnant ablation with radioactive iodine [Citation64,Citation65]. However, a lobectomy is also accepted for patients with an indeterminate nodule on cytology or single microcarcinoma of less than 1 cm with no other suspicious history or findings. Preoperative ultrasonography with FNA aspiration of suspicious lymph nodes is advised [Citation66]. Age by itself should not preclude patients from having surgery, since older population tends to have more aggressive cancers.
Radioactive iodine ablation and scan is used to eliminate post-surgical thyroid remnant and to identify previously undiagnosed disease. Risk stratification of the individual patient should be taken into account. Numerous studies have shown a significant reduction in disease recurrence and cause-specific mortality [Citation67–69]. Suppressive doses of thyroxine are recommended to maintain serum TSH at less than 0.1 mU/l in patients with more advanced tumours and 0.1–0.4 mU/l in patients with less advanced disease. However, thyroxine suppression therapy may be equivalent to subclinical thyrotoxicosis, which may trigger atrial fibrillation in older population. Cappola et al. designed a prospective cohort study, which included people aged 65 years or older. Patients with subclinical hyperthyroidism had a greater incidence of atrial fibrillation over a 13-year follow-up than the euthyroid group, with an adjusted hazard ratio of 1.98 [Citation70]. Follow-up management of differentiated thyroid cancer includes physical exam, whole body scan, neck ultrasound and measurement of serum thyroglobulin and thyroglobulin antibodies.
Unlike differentiated thyroid carcinoma, radioiodine treatment is not indicated in medullary thyroid carcinoma. Multiple endocrine neoplasia type 2 needs to be ruled out by RET testing, serum calcium, and 24-h urinary excretion of metanephrines and catecholamines in all patients with medullary thyroid cancer. Pheochromocytoma needs to be treated prior to medullary thyroid cancer. Total thyroidectomy with central neck compartment dissection is indicated in all patients due to high frequency of bilateral disease. More extensive surgical approach may be appropriate for more invasive tumours [Citation39,Citation71]. External beam radiation may be considered for patients with high risk of regional recurrence [Citation72]. Maintenance of normal serum TSH is recommended postoperatively for medullary thyroid carcinoma patients. Serum concentrations of calcitonin and carcinoembryonic antigen should be followed [Citation71].
Treatment of anaplastic thyroid cancer is mostly palliative. Therapy options include surgery, external beam radiation therapy, chemotherapy and tracheostomy. All options should be carefully evaluated based on the ability to promote quality of life and prolong survival. The aggressive nature of this disease results in a high rate of recurrence despite of these therapies [Citation73].
Appropriate treatment for patients with primary thyroid lymphoma depends on histologic subtype. Surgical treatment is no longer indicated. Early stage tumour subtypes may be treated with local therapies while the more aggressive lymphomas are treated with R-CHOP (rituximab, cyclophosphamide, adriamycin, vincristine, and prednisone) [Citation60]. A study of 1222 elderly patients aged 61–80 years showed significantly improved survival with six cycles of R-CHOP [Citation74].
Conclusion
Thyroid carcinoma is the most common endocrine malignancy. It includes tumour types that range from localised papillary carcinomas to lethal anaplastic disease. Even though thyroid cancer can occur in any age group, its aggressiveness increases significantly in older patients, particularly older males. Early diagnosis and urgent aggressive treatment are important for aging patients with thyroid cancer. Regular physical examinations and evaluation of any neck mass are indicated. Age by itself is not a contraindication to surgery and appropriate thyroid cancer management. Coordination of care should be done by a multidisciplinary team to optimise quality of life and survival of thyroid cancer patients.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Yancick R, Ries LA. Cancer in older persons. Cancer 1994;74:1995–2003.
- Audisio RA, Repetta L, Zagonel V. Cancer in the elderly. In: UICC manual of clinical oncology. Pollock RE, Doroshow JH, editors. 8th ed. New Jersey: Wiley-Liss, 2004: (39):849–873.
- Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 2009;115:3801–3807.
- Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006;295:2164–2167.
- Monganti S, Ceda GP, Saccani M, et al. Thyroid disease in the elderly: Sex-related differences in clinical expression. J Endocrinol Invest 2005;28(11 Suppl Proceedings):101–104.
- Mazzaferri E. Management of a solitary thyroid nodule. NEJM 1993;328:553–559.
- Schlumberger MJ, Filetti S, Hay ID. Nontoxic diffuse and nodular goiter and thyroid neoplasia. 11th ed. Kronenberg: Williams Textbook of Endocrinology; 2008. Chapter 13.
- Frates MCFr, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, Cronan JJ, Doubilet PM, Evans DB, Goellner GR, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology 2005;237:794–800.
- Papini E, Guglielmi R, Bianchini A, Crescenzi A, Toccogna S, Nardi F, Panunzi C, Rinaldi R, Toscano V, Pacella C. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab 2002; 87:1941–1946.
- Lin JD, Chao TC, Huang BY, Chen ST, Chang HY, Hsueh C. Thyroid cancer in the thyroid nodules evaluated by ultrasonagraphy and fine-needle aspiration cytology. Thyroid 2005;15:708–717.
- Belfiore A, La Rosa GL, La Porta GA, et al. Cancer risk in patients with cold thyroid nodules: relevance of iodine intake, sex, age, and multinodularity. Am J Med 1992;93:363–369.
- Blum M, Rothschild M. Improved nonoperative diagnosis of the solitary “cold” thyroid nodule: surgical selection based on risk factors and three months of suppression. JAMA 1980; 243:242–245.
- Welker MJ, Orlov D. Thyroid nodules. Am Fam Physician 2003;67:559–566.
- Lin JD, Chao TC, Chen ST, Weng HF, Lin KD. Characteristics of thyroid carcinomas in aging patients. Eur J Clin Invest 2000;30:147–153.
- Tuttle M, Leboeuf R, Martorella A. Papillary thyroid cancer: monitoring and therapy. Endocrinol Metab Clin 2007;36: 753–778.
- Kilfoy BA, Devesa SS, Ward MH, Zhang Y, Rosenberg PS, Holford TR, Anderson WF. Gender is an age-specific effect modifier for papillary cancers of the thyroid gland. Cancer Epidemiol Biomarkers Prev 2009;4: 1092–1100.
- Arturi F, Russo D, Giuffrida D, Ippolito A, Perrotti N, Vigneri R , Filetti S. Early diagnosis by genetic analysis of differentiated thyroid cancer metastases in small lymph nodes. J Clin Endocrinol Metab 1997;82:1638–1641.
- Qubain S.W, Nakano S, Baba M, Takao S, Aikou T. Distribution of lymph node micrometastasis in pN0 well-differentiated thyroid carcinoma. Surgery 2002;131:249–256.
- McConahey WM, Hay ID, Woolner LB, van Heerden JA, Taylor WF. Papillary thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc 1986;61:978–996.
- Sebastian SO, Gonzalez JM, Paricio SP, Perez S, Flores P, Madrona P, Romero RP, Tebar FJ. Papillary thyroid carcinoma: prognostic index for survival including the histological variety. Arch Surg 2000;135:272–277.
- Mazaferri EL. Papillary thyroid carcinoma: factors influencing prognosis and current therapy. Semin Oncol 1987;14;315–332.
- Goldballe C, Asschenfeld P, Sorensen JA, Sorensen MW, Jorgensen K. Papillary thyroid carcinoma: correlations between prognosis, age, and clinicopathological and histomorphological findings. Laryngoscope 1994;104: 747–751.
- Sautter-Bihl M, Raub J, Hetzel-Sesterheim M, Heinze HG. Differentiated thyroid cancer. Prognostic factors and influence of treatment on the outcome in 441 patients. Strahlenther Onkol 2001;177:125–131.
- Hawk WA, Hazzard JB. The many appearances of papillary carcinoma of the thyroid. Clev Clin Q 1976;43:207–216.
- Machens A, Hauptmann S, Dralle H. Disparities between male and female patients with thyroid cancers: sex difference or gender divide? Clin Endocrinol 2006;65:500–505.
- Levi F, Randimbison L, Te VC, La Vecchia C. Thyroid cancer in Vaud, Switzerland: an update. Thyroid 2002;12:163–167.
- Mazzaferri E, Lhiang S. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 1994;97:418–428.
- Correra P, Chen VW. Endocrine gland cancer. Cancer 1995;75(Suppl):338–352.
- Gupta KL. Neoplasm of the thyroid gland. Clin Geriatr Med 1995;11:271–290.
- Grebe SKG, Hay ID. Follicular thyroid cancer. Endocrinol Metab Clin North Am 1996;24:761–801.
- Phitayakorn R, Mchenry C. Follicular and Hurthle cell carcinoma of the thyroid gland. Surg Oncol Clin N Am 2006;15:603–623.
- Woolner LB. Thyroid carcinoma: pathologic classification with data on prognosis. Semin Nucl Med 1971;1:481–502.
- Schneider AB, Sarne DH. Long-term risks for thyroid cancer and other neoplasms after exposure to radiation. Natl Clin Pract Endocrinol Metab 2005;1:82.
- Mazzaferri E. Thyroid Cancer. Principles and practice of endocrinology and metabolism. 3rd ed, 2001. Chapter 40, Lippincott Williams and Wilkins, Philadelphia.
- Tuttle M, Lemar H, Burch H. Clinical features associated with an increased risk of thyroid malignancy in patients with follicular neoplasia by fine-needle aspiration. Thyroid 1998;8: 377–383.
- Crile G, Pontius KI, Hawk WA. Factors influencing the survival of patients with follicular carcinoma of the thyroid gland. Surg Gynecol Obstet 1985;160:409–413.
- Brandi ML, Gagel RF, Angeli A, Bilezikean JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 2001;86:5658–5671.
- Kebebew E, Ituarte PH, Siperstein AE, Duh QY, Clark OH. Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 2000;88:1139–1148.
- Sherman Steven. Thyroid carcinoma. Lancet 2003;361:501–511.
- Elisei R, Bottici V, Luchetti F, Coscio G, Romei C, Grasso L, Miccoli P, Iaconni P, Basolo F, Pinchera A. Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10,864 patients with nodular thyroid disorders. J Clin Endocrinol Metab 2004;89:163–168.
- Hahm JR, Lee MS, Min YK, Lee MK, Kim KW, Nam SJ, Yang JH, Chung JH. Routine measurement of serum calcitonin is useful for early detection of medullary thyroid carcinoma in patients with nodular thyroid diseases. Thyroid 2001;11:73–80.
- Redding AH, Levine SN, Fowler MR. Normal preoperative calcitonin levels do not always exclude medullary thyroid carcinoma in patients with large palpable thyroid masses. Thyroid 2000;10:919–922.
- Saad MF, Ordonez NG, Rashid RK, Guido JJ, Hill SC, Hickey RC, Samaan NA. Medullary carcinoma of the thyroid. Medicine 1984;63:319–342.
- Scopsi L, Sampietro G, Boracchi P, Del Bo R, Gullo M, Placucci M, Pilotti S. Multivariate analysis of prognostic factors in sporadic medullary carcinoma of the thyroid. A retrospective study of 109 consecutive patients. Cancer 1996;78:2173–2183.
- Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the US, 1985–1995. Cancer 1998;83:2638–2648.
- Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol 2006;13:453–464.
- Hadar T, Mor C, Shvero J, Levy R, Segal K. Anaplastic carcinoma of the thyroid. Eur J Surg Oncol 1993;19:511–516.
- Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma: a population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer 1997;79:564–573.
- Ain KB. Anaplastic thyroid carcinoma: a therapeutic challenge. Semin Surg Oncol 1999;16:64–69.
- Giuffrida D, Gharib H. Anaplastic thyroid carcinoma: current diagnosis and treatment. Ann Oncol 2000;11:1083–1089.
- Haigh PI, Ituarte PH, Wu HS, Treseler PA, Posner MD, Quivey JM, Duh QY, Clark OH. Completely resected anaplastic thyroid carcinoma combined with adjuvant chemotherapy and irradiation is associated with prolonged survival. Cancer 2001;91:2335–2342.
- Kim TY, Kim KW, Jung TS, Kim JM, Kim SW, Chung K, Kim EY, Gong G, OH YL, Cho SY, et al. Prognostic factors for Korean patients with anaplastic thyroid carcinoma. Head Neck 2007;29:765–772.
- Pierie JP, Muzikansky A, Gaz RD, Faquin WC, Ott MJ. The effect of surgery and radiotherapy on outcome of anaplastic thyroid carcinoma. Ann Surg Oncol 2002;9:57–64.
- Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A. Anaplastic thyroid carcinoma treatment outcome and prognostic factors. Cancer 2005;103:1330–1335.
- Tan RK, Finley RK, Driscoll D, Bakamjian V, Hicks WL, Shedd DP. Anaplastic carcinoma of the thyroid: a 24-year experience. Head Neck 1995;17:41–47.
- Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. Prognostic factors and therapeutic strategy for anaplastic carcinoma of the thyroid. World J Surg 2001;25:617–622.
- Ansell SM, Grant CS, Habermann TM. Primary thyroid lymphoma. Semin Oncol 1999;26:316–323.
- Derringer GA, Thompson LD, Frommelt RA, Bijwaard KE, Hefess CS, Abbondanzo SL. Malignant lymphoma of the thyroid gland: a clinicopathologic study of 108 cases. Am J Surg Pathol 2000;24:623–639.
- Belal AA, Allam A, Kandil A, Husseiny G, Khafaga Y, Rajhi A, Ahmed G, Gray A, Ajarim D, Schultz H. Primary thyroid lymphoma: a retrospective analysis of prognostic factors and treatment outcome for localized intermediate and high grade lymphoma. Am J Clin Oncol 2001;24:299–305.
- Graff-Baker A, Sosa JA, Roman SA. Primary thyroid lymphoma: a review of recent developments in diagnosis and histology-driven treatment. Curr Opin Oncol 2010;22:17–22.
- Holm LE, Blomgren H, Lowhagen T. Cancer risks in patients with chronic lymphocytic thyroiditis. N Eng J Med 1985;312:601–604.
- Hwang YC, Kim TY, Kim WB, Shong YK, Yi KH, Shong M, Jo YS, Kim WS, Chung JH. Clinical characteristics of primary thyroid lymphoma in Koreans. Endocr J 2009;56:399–405.
- Graff-Baker A, Roman SA, Thomas DC, Udelsman R, Sosa JA. Prognosis of primary thyroid lymphoma: demographic, clinical and pathologic predictors of survival in 1,408 cases. Surgery 2009;146: 1105–1115.
- Mazzaferri EL, Kloos RT. Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab 2001;86:1447–1463.
- Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W. European consensus for the management of patients with differentiated thyroid cancer of the follicular epithelium. Eur J Endocrinol 2006;154:783–803.
- Cooper D, Doherty G, Haugen B, et al. Revised thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19: 1167–1214.
- Mazzaferri EL, Jhiang SM. Differentiated thyroid cancer long-term impact of initial therapy. Trans Am Clin Climatol Assoc 1994;106:151–168.
- Samaan NA, Shultz PN, Hickey RC, Goepfert H, Haynie TP, Johnston DA, Ordonez NG. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab 1992;75:714–720.
- Sawka AM, Brierley JD, Tsang RW, et al. An updated systematic review and commentary examining the effectiveness of radioactive iodine remnant ablation in well-differentiated thyroid cancer. Endocrinol Metab Clin North Am 2008;37:457–480.
- Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA 2006;295:1033–1041.
- Kloos RT, Eng C, Evans DB, et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 2009;19:565–612.
- Brierley J, Tsang R, Simpson WJ, et al. Medullary thyroid cancer: analysis of survival and prognostic factors and the role of radiation therapy in local control. Thyroid 1996;6:305–310.
- Neff RL, Farrar WB, Kloos RT. Anaplastic thyroid cancer. Endocrinol Metab Clin N Am 2008;37:525–538.
- Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 2008;9:105–116.