Abstract
Objective. Physical activity (PhA) has proven to be a protective factor for normal erectile function. The aim of this study was to evaluate the effects of a standard protocol of aerobic PhA on quality of erectile dysfunction (ED) in patients with arterial ED.
Materials and methods. Fifty patients (48–62 years) were selected and underwent to standard protocol of aerobic PhA: 150 min of moderate intensity aerobic activity per week (group A). Twenty patients, matched aged, with vascular ED who did not accept to undergo the standard PhA's protocol, represented the control group. All patients were evaluated, by IIEF-5 questionnaire administration, penile eco color doppler and flow-cytometric analysis for detection of serum concentrations of original immunophenotype endothelial progenitor cells (EPCs) = CD45neg/CD34pos/CD144pos and endothelial microparticles (EMPs) = CD45neg/CD34neg/CD144pos.
Results. After 3 months, group A showed IIEF 5 score and peak systolic velocity significantly higher (p < 0.05) compared to controls, and significantly lower values (p < 0.05) of acceleration time, in addition serum concentrations of EPCs and EMPs were significantly lower (p < 0.05) in group A compared to controls.
Conclusions. PhA improves quality of arterial ED, without other pharmacological approach, probably by reduced endothelial apoptosis. This study characterises the study of endothelial dysfunction by new cell circulating markers.
Introduction
There is now a wealth of sophisticated epidemiological evidence to demonstrate that physical activity (PhA) is associated with reduced risk of coronary heart disease, obesity, type 2 diabetes and other chronic diseases and conditions. Causal relationships between PhA and cardiovascular disease, type 2 diabetes, colon cancer and all-cause mortality have been recognised for some time. More recently, the pertinent issue has been the dose–response relationship between PhA and health: What is the minimum dose of activity associated with health and well-being? What doses of activity offer greater health benefits? [Citation1].
Physical activity end erectile dysfunction
PhA has proven to be a protective factor for normal erectile function in numerous epidemiological studies. In a recent study, a total of 60 patients complaining of erectile dysfunction (ED) were studied. Patients were assessed at baseline and after 3 months of study treatment, in this study at baseline, patients were randomised to receive phosphodiesterase type 5 inhibitor (PDE5i) alone (group A) or PDE5i plus regular (≥3 h/week), aerobic, non-agonistic PhA (group B). All subjects completed the International Index of Erectile Function (IIEF-15) questionnaire. IIEF restoration of ED occurred in 77.8% (intervention group) versus 39.3% (control) (p < 0.004). The IIEF-15 score resulted in statistical improvement in intervention group in all the domains but one (orgasm): erectile function, 24.7 versus 26.8 (p = 0.003); confidence (Q15), 3.53 versus 4.07 (p = 0.006); sexual desire, 6.46 versus 7.18 (p = 0.028); intercourse satisfaction, 9.85 versus 11.25 (p = 0.001); total satisfaction, 7.17 versus 8.07 (p = 0.009); total score, 56.2 versus 61.07 (p = 0.007). PhA was the only independent variable for normal erection (p = 0.010) (95% confidence interval [CI]: 0.036–0.643), higher sexual satisfaction (p = 0.022) (95% CI: 0.084–0.821) and normal total IIEF-15 score (p = 0.023) (95% CI: 0.85–0.837) [Citation2].
In an evaluation of 3.941 adult men (age ≥20 years) logistic regression analyses were used to examine the relative odds of ED association with PhA. PhA level was divided into active (≥150 min/week), moderately active (30–149 min/week) and inactive (<30 min/week) categories. Moderately active or inactive men had an approximately 40–60% greater odds of ED compared with active men. The logistic regression model showed that PhA level were independently associated with a greater odds of ED. Moderate-intensity PhA (≥150 min/week) is associated with the maintenance of proper erectile function [Citation3].
In a other study of Esposito et al., total of 209 subjects were randomly assigned to one of the two treatment groups. The 104 men randomly assigned to the intervention programme received detailed advice about how to increase the PhA. The 105 subjects in the control group were given general information about their level of PhA. Erectile function score improved in the intervention group. At baseline, 35 subjects in the intervention group and 38 subjects in the control group had normal erectile function (34% and 36%, respectively). After 2 years, these figures were 58 subjects in the intervention group and 40 subjects in the control group, respectively (56% and 38%, p = 0.015). There was a strong correlation between the success score and restoration of erectile function [Citation4].
Physical activity and endothelial dysfunction
The mechanisms by which exercise improves endothelial function are not fully clarified. Several mechanisms have been proposed to explain the positive effect of exercise on the disease progression. They include the decrease in cytokine production by the adipose tissue, skeletal muscles, endothelial cells and blood mononuclear cells, and also, the increase in the bioavailability of nitric oxide, antioxidant defences and regenerative capacity of endothelium [Citation5].
Mobilisation of bone marrow-derived endothelial progenitor cells (EPC) might explain exercise-induced improvement of endothelial function, in a recent study, EPC were quantified by flow cytometry and cell culture in 25 healthy volunteers undergoing three protocols of running exercise. Intensive running, defined as 30 min at 100% of the velocity of the individual anaerobic threshold (IAT; approximately 82% maximal oxygen consumption; VO2max), as well as moderate running with 30 min at 80% of the velocity of the IAT (approximately 68% VO2max), increased circulating EPC numbers to 235% ± 93% and 263% ± 106% of control levels, respectively [Citation6].
A sedentary lifestyle has adverse effects on the cardiovascular system, including impaired endothelial functions. In a recent study on healthy men to 7 days of dry immersion (DI), the authors have investigated endothelial properties before, during and after 7 days of DI involving eight subjects. Microcirculatory functions were assessed with laser doppler in the skin of the calf and basal blood flow and endothelium-dependent and independent vasodilation were studied. Also plasma levels of microparticles, a sign of cellular dysfunction, and soluble endothelial factors, reflecting the endothelial state were measured. Basal flow and endothelium-dependent vasodilation were reduced by DI (22 ± 4 vs. 15 ± 2 arbitrary units and 29% ± 6% vs. 12% ± 6%, respectively, p < 0.05), and this was accompanied by an increase in circulating endothelial microparticles (EMPs), which was significant on day 3 (42 ± 8 vs. 65 ± 10 EMPs/microliter, p < 0.05), whereas microparticles from other cell origins remained unchanged. Plasma soluble VEGF decreased significantly during DI, whereas VEGF receptor 1 and soluble CD62E were unchanged, indicating that the increase in EMPs was associated with a change in antiapoptotic tone rather than endothelial activation. This study showed that extreme physical inactivity in humans induced by 7 days of DI causes microvascular impairment with a disturbance of endothelial functions, associated with a selective increase in EMPs. Microcirculatory endothelial dysfunction might contribute to cardiovascular deconditioning as well as to hypodynamia-associated pathologies [Citation7].
A recent cross-sectional multicenter study with six research groups was undertaken, the purpose of this study was to analyse the relationship of PhA to the circadian pattern of blood pressure, central and peripheral blood pressure, pulse wave velocity, carotid intima-media thickness and biological markers of endothelial dysfunction in active and sedentary individuals without arteriosclerotic disease. Determining that sustained PhA and the change from sedentary to active improve circadian pattern, arterial elasticity and carotid intima-media thickness may help to propose lifestyle intervention programmes. These interventions could improve the cardiovascular risk profile in some parameters not routinely assessed with traditional risk scales. From the results of this study, interventional approaches could be obtained to delay vascular aging with physical exercise [Citation8].
New markers of endothelial dysfunction
ED may be evaluated in many ways and recently this has been done by estimating the number of circulating endothelial precursor cells (EPCs) and endothelial microparticles (EMPs) [Citation9]. EPCs are progenitor cells similar to the embryonic angioblast; they derive from the mesoderm and have a common precursor with the haematopoietic stem cells (HSC), the emangioblast. EPCs may also originate from trans-differentiated monocyte/macrophages [Citation10]. The first EPC phenotype was defined by the presence of the following antigens in blood cells: CD34, CD133 and VEGFR2 (or KDR) [Citation11]. However, recent observations suggest that this EPC phenotype is rich in haematopoietic progenitor cells (HPC) and expresses the panleukocyte antigen CD45. Moreover, these cells have been reported to be unable to form endothelial colonies in-vitro. A closer look at the EPC biology indicates a progressive loss of CD133 and CD34 antigens and the expression of CD31, VE-cadherin (CD144) and Vwf, following their mobilisation into the general circulation [Citation12,Citation13]. Thus, the absence of CD45 and the concomitant presence of CD34 and CD144 antigens assure that these cells are true endothelial stem/progenitor cells [Citation13–16]. On this basis, we chose to evaluate the following EPCs phenotype: CD45neg/CD34pos/CD144pos.
In addition to EPCs, EMPs may be found in the general circulation. Cellular microparticles (MPs) are fragments of the plasma membrane that are shed by virtually all cells undergoing stress conditions, including cell activation and apoptosis. Since the description of the ‘platelet dust’ [Citation17] numerous studies have reported the presence of subcellular vesicles in centrifuged plasma. Although long considered to be cellular debris, blood MPs are more recently considered reflective of cellular stimulation, activation and degeneration/apoptosis. By general consensus, MPs are small in size (≤1.5 μm). EMPs are released as consequence of endothelial dysfunction, atherogenesis and endothelial apoptotic processes [Citation18].
There are no data on the benefits of a standard protocol of aerobic PhA on vascular ED, and in particular, no studies have assessed endothelial function by evaluation of circulating endothelial progenitors cells and endothelial microparticles, after PhA in these patients.
Based on these findings, the aim of this study was to evaluate the effects of a standard protocol of aerobic PhA on quality of ED in selected men with arterial ED, without other specific treatment for ED.
Patients and methods
Patient selection
Fifty patients with arterial ED were enrolled, mean age: 57.3 ± 0.5 years (range: 48–62). The diagnosis of arterial ED was made when all the following criteria were fulfilled: (a) international index of erectile function (IIEF-5) score <21 [Citation19]; (b) cavernousal artery peak systolic velocity (PSV) <30 cm/sc, 10 and 20 min after the intracavernousal injection of alprostadil (20 μg) by echo color Doppler [Citation20]; (c) acceleration time >110 ms [Citation21].
Exclusion criteria: coronary artery disease, carotid and lower limbs atherosclerosis, smokers, diabetics.
All 50 patients were underwent to aerobic phisical activity's protocol () and observation of the Mediterranean diet's principles () explained by the specialist at the beginning and during each monthly conventional visit and represented the group A.
Table I. Protocol of aerobic physical activity and Mediterranean diet's principles observed by patients during the study.
Twenty patients, mean age: 55.8 ± 1.2 years (range: 46–64) were excluded from aerobic PhA protocol for rejection and represented control group, these patients observed only the principles of the Mediterranean diet.
All patients underwent at baseline and after 3 months to dynamic penile echo colour Doppler and blood withdrawal (100 μl), for EPC and EMP measurements by flow cytometry. In addition to, all was administered IIEF-5 questionnaire. The study was approved by the internal Institutional Review Board and an informed written consent was obtained from each patient.
Blood EPC and EMP determination
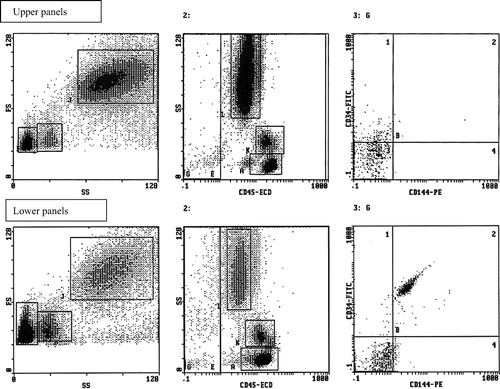
EPCs and EMPs evaluation was performed in blood (100 μl) following incubation in erythrocyte lysing solution (Versalyse, IL, Milan, Italy) for 1 min. The suspension was then washed twice with phosphate buffer solution (PBS), centrifuged and the pellet was rapidly incubated in PBS containing the following monoclonal antibodies: anti-CD45 (10 μl), anti-CD34 (20 μl) and anti-CD144 (20 μl), respectively labelled with r-phycoerythrin covalently bound to Texas red (ECD), fluorescein isothiocyanate (FITC) and r-phycoerythrin (PE), at room temperature for 20 min. Each antibody was tested with the isotypic control. Each sample was analysed by flow cytometry (EPICS XL, Coulter Electronics, IL, Milan, Italy) using the following gating strategy (). Histograms 1 report the forward versus side scatter dot plot: 3 different cell populations can be identified; gate F: lymphocytes; gate I: monocytes; gate L: polymorphonuclear cells; Histograms 2 report the CD45pos (E) and CD45neg (G) cells; Histograms 3 report only the CD45neg cells (gate G) subdivided according to the staining with CD34 and CD144 antigens. EPCs were defined as CD45neg/CD34pos/CD144pos, whereas EMPs as CD45neg/CD34neg/CD144pos events. Flow cytometric analysis was conducted for 600 s or 100,000 events (cells) whichever occurred first.
Figure 1. Representative flow cytometric scattergrams showing the gating strategy used. Upper panels, after PhA standard aerobic protocol in patients with ED; lower panels, before PhA aerobic protocol. Both histograms 1 report the forward versus side scatter dot plot: three different cell populations can be identified (gate F: lymphocytes; gate I: monocytes; gate J: polymorphonuclear cells). Both histograms 2 report the CD45pos (E) and CD45neg (G) cells. Both histograms 3 report only the CD45neg cells (gate G) subdivided according to the expression of CD34 and CD144 antigen staining. EPCs were defined as CD45neg/CD34pos/CD144pos, whereas EMP CD45neg/CD34neg/CD144pos.
Statistical analysis
Results are shown as mean ± SEM. EPC and EMP values were log-transformed before being analysed. Statistical analysis was conducted by one-way analysis of variance (ANOVA) followed by the Duncan Multiple range test or unpaired Student's t-test, as appropriate. Statistical analysis was conducted using SPSS 10.0 for Windows. A p-value lower than 0.05 was accepted as statistically significant.
Results
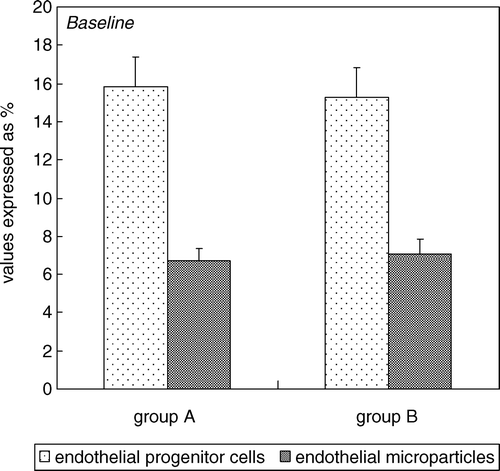
No statistically significant difference (p value >0.05) in age between the two groups was observed. At baseline, no statistically significant difference between the two groups, relatively to the following parameters: IIEF-5 score, peak systolic velocity, acceleration time and relatively to others vascular parameters as: end diastolic velocity, resistance index, intimal cavernous thickness (). In addition, no statistically significant difference (p value >0.05) between the two groups for the following metabolic characteristics: body mass index, total cholesterol, HDL, triglycerides, blood pressure detected during the enrollment visit (). Finally, at baseline, no statistically significant difference (p value >0.05) between the two groups relatively serum EPCs (15.8% ± 1.4% vs. 15.3% ± 2.3%) and EMP concentration (6.7% ± 0.4% vs. 7.1% ± 1.1%) ().
Table II. Penile dynamic echo-color doppler parameters, IIEF-5 score and metabolic features (mean ± SEM) of the patients (group A and group B) with arterial erectile dysfunction at baseline and after three months of standard aerobic protocol of physical activity.
Figure 2. Percentage of circulating endothelial progenitor cells (immunophenotype CD45neg/CD34pos/CD144pos) and percentage of circulating endothelial microparticles (immunophenotype CD45neg/CD34neg/CD144pos) in all patients with ED at baseline (group A compared to group B).
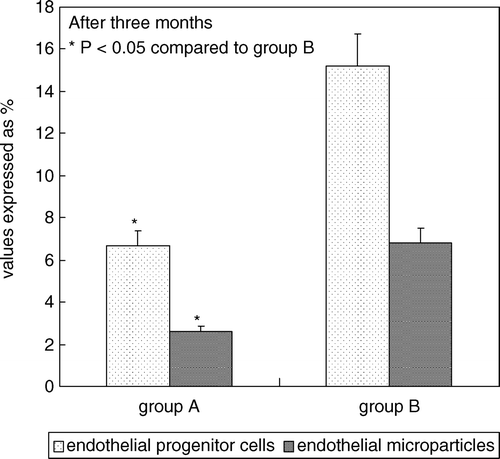
After 3 months, group A showed mean IIEF-5 score significantly higher (p value <0.05) compared to themselves at baseline and group B () and showed statistically significant difference (p value <0.05) relatively to all parameters investigated (peak systolic velocity, acceleration time, body mass index, total cholesterol, HDL, triglycerides, blood pressure detected during second visit), compared to themselves at baseline and group B (). Finally, group A showed mean serum concentrations of EPCs (6.7% ± 1.2% vs. 15.2% ± 2.8%) and EMP (2.6% ± 0.46% vs. 6.8% ± 0.9%) significantly lower (p value <0.05) compared to themselves at baseline and group B ().
Figure 3. Percentage of circulating endothelial progenitor cells (immunophenotype CD45neg/CD34pos/CD144pos) and percentage of circulating endothelial microparticles (immunophenotype CD45neg/CD34neg/CD144pos) in all patients with ED after 3 months of PhA standard aerobic protocol (group A compared to group B).
Discussion
The study evaluated the benefits induced by aerobic PhA on the severity of ED in a setting of selected middle-aged patients with arterial ED and found a significant improvement in all vascular and metabolic parameters analysed, in particular a reduction of apoptosis and repair endothelial assessed by original immunophenotype of circulating EPC and MPE.
The results of this study shows, in agreement with previous articles by other authors, that PhA improves the severity of ED; however, with some important points for originality: a clear clinical setting of ED patients analysed, a well-defined approach of PhA and finally a new methodological model for the study of reparative and apoptotic endothelial function.
ED is experienced at least some of the time by most men who have reached 45 years of age, and it is projected to affect 322 million men worldwide by 2025. The prevalence of ED is high in men of all ages and increases greatly in the elderly [Citation24].
ED's severity and prevalence both increase with aging: since ED is a symptom, physicians should diagnose underlying pathologies that might lead to it instead of focussing on finding a viable treatment. Cardiovascular alterations occur in the elderly and might lead to ED because of penile blood flow impairment: diabetes, smoking and sedentary life-style, being risk factors for vascular pathologies, can affect erectile function. Metabolic syndrome and psychological factors are highly prevalent in aging men and might be other important determinants of ED. Drugs play a role in the pathogenesis of ED, as they can alter hormonal or vascular mechanics needed for achieving or maintaining erection. Alterations in penile vessels can be observed in the elderly: lack of androgens might lead to a reduction of smooth muscle cells content in the penis and an increase in the caliber of vascular spaces [Citation25].
About 60% of the elderly population expresses their interest for maintaining sexual activity. Although aging and functional decline may affect sexual function, when sexual dysfunction is diagnosed, physicians should rule out disease or side effects of medications. Common disorders related to sexual dysfunction include cardiovascular disease, diabetes, lower urinary tract symptoms and depression. Early control of cardiovascular risk factors may improve endothelial function and reduce the occurrence of ED. Treating those disorders or modifying lifestyle-related risk factors (eg obesity) may help prevent sexual dysfunction in the elderly [Citation26].
Physical inactivity negatively impacts on erectile function, and experimental and clinical exercise interventions have been shown to improve sexual responses and overall cardiovascular health. Mediterranean-style diets and a reduction in caloric intake have been found to improve erectile function in men with the aspects of the metabolic syndrome. In addition, both clinical and experimental studies have confirmed that combining the two interventions provides additional benefit to erectile function, likely via reduced metabolic disturbances (e.g. inflammatory markers, insulin resistance), decreased visceral adipose tissue and improvement in vascular function (e.g. increased endothelial function) [Citation27].
A recent study on 674 men aged 45–60 year that included a urological physical examination, medical history and assessment of testosterone (T) and sex hormone-binding globulin showed a positive correlation between the IIEF-5 and the Paffenbarger score (specific questionnaire for self-reported PhA levels) (r = 0.164, p < 0.001). The IIEF-5 score increased with an increasing Paffenbarger score (PhA index) up to a level of 4000 kcal/week. T revealed a trend to a significant impact on the IIEF-5 score but showed no association with the Paffenbarger score. The risk of severe ED was decreased by 82.9% for males with PhA of at least 3000 kcal/week compared with males with PhA under 3000 kcal/week (OR = 0.171, p = 0.018) [Citation28].
BMI and PhA independently and differentially affected ED risk. BMI had greatest influence with low physical activity, and PhA exerted greatest influence when BMI was high. A population representative cross-sectional analytic study of ED in Hong Kong, with two-stage stratified random sampling and face-to-face interviews conducted by trained interviewers with structured questionnaires. Study subjects were 1506 men aged 26–70. In this study, age, PhA and general psychological distress were independently associated with ED after multivariate adjustments. An U-shaped relationship between BMI and ED was observed only among men with no exercise (<once/week): BMI <18.5 (OR = 2.99; 95% CI: 1.01–8.86), 18.5–19.9 (OR = 2.66; 95% CI: 1.04–6.79), 20.0–20.9 (OR = 1.37; 95% CI: 0.49–3.79), 22.0–22.9 (OR = 1.36; 95% CI: 0.58–3.17), 23.0–24.9 (OR = 1.66; 95% CI: 0.70–3.93), ≥25.0 (OR = 2.47; 95% CI: 1.08–5.67) using BMI 21.0–21.9 as reference, adjusted for age and smoking status. Being physically active (≥1000 kcal/week) only reduced the risk of ED (OR = 0.40, 95% CI: 0.16–0.95) in men who were obese, adjusted for age smoking status and BMI [Citation29].
Another recent study was conducted from October 2000 to October 2003 at a university hospital in Italy. The 55 men randomly assigned to the intervention group received detailed advice about how to achieve a loss of 10% or more in their total body weight by reducing caloric intake and increasing their level of physical activity. Men in the control group (n = 55) were given general information about healthy food choices and exercise. Erectile function score, levels of cholesterol and triglycerides, circulating levels of interleukin 6, interleukin 8 and C-reactive protein and endothelial function as assessed by vascular responses to l-arginine. After 2 years, body mass index decreased more in the intervention group (from a mean [SD] of 36.9 [2.5] to 31.2 [2.1]) than in the control group (from 36.4 [2.3] to 35.7 [2.5]) (p < 0.001), as did serum concentrations of interleukin 6 (p = 0.03) and C-reactive protein (p = 0.02). The mean (SD) level of PhA increased more in the intervention group (from 48 [Citation10] to 195 [36] min/week; p < 0.001) than in the control group (from 51 [Citation9] to 84 [Citation28] min/week; p < 0.001). The mean (SD) IIEF score improved in the intervention group (from 13.9 [4.0] to 17 [Citation5]; p < 0.001), but remained stable in the control group (from 13.5 [4.0] to 13.6 [4.1]; p = 0.89). Seventeen men in the intervention group and three in the control group (p = 0.001) reported an IIEF score of 22 or higher. In multivariate analyses, changes in body mass index (p = 0.02), PhA (p = 0.02) and C-reactive protein (p = 0.03) were independently associated with changes in IIEF score [Citation30].
PhA and EPC/MPE serum concentrations
The results of recent study suggest that finishing a marathon race will lead to an inflammatory response and down-regulation of circulating haematopoietic stem cells. With respect to EPCs no change is observed, which may be because of a greater differentiation of the remaining CD34 cells towards EPCs. Sixty-eight healthy marathon runners (age: 57 ± 6 years) were included in this study. Blood cell counts were evaluated by standard methods and circulating progenitor cells before and immediately after the race were quantified by fluorescence-activated cell sorter (FACS). Vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF) was quantified by enzyme-linked immunosorbent assay. A marathon race led to a significant increase in white blood cell count (5283 ± 155 vs. 13,706 ± 373 cells/microliter; p < 0.001). Fluorescence-activated cell sorter analysis revealed a significant decrease of CD34 cells (1829 ± 115 vs. 1175 ± 75 cells/ml blood; p < 0.0001), CD117 cells (2478 ± 245 vs. 2193 ± 85 cells/ml blood; p < 0.05) and CD133 cells (3505 ± 286 vs. 2239 ± 163 cells/ml blood; p < 0.001). No significant change was observed for EPCs defined as CD34/VEGF-R2 cells (117 ± 8 vs. 128 ± 9 cells/ml blood; p = 0.33). With respect to VEGF, a significant down-regulation was evident directly after the race (48.9 ± 8.0 vs. 34.0 ± 7.5 pg/ml; p < 0.05), whereas no change was obvious in EGF levels [Citation31].
A maximal bout of exercise induces a significant shift in CD34+ cells toward CD34+/KDR+ cells; this response was larger in subjects with a less favourable lipid profile. In this study, healthy subjects (group 1, n = 11; group 2, n = 14) performed a symptom-limited cardiopulmonary exercise test on a bicycle ergometre. Numbers of CD34+/kinase insert domain receptor (KDR)+ cells were determined by flow-cytometric analysis, either after magnetic separation of CD34+ cells (group 1) or starting from whole blood (group 2). Serum concentrations of VEGF and NO metabolites were measured by using ELISA. Following exercise, EPC increased by 76% (15.4 ± 10.7 cells/ml vs. 27.2 ± 13.7 cells/ml; p = 0.01) in group 1 and by 69% in group 2 (30.9 ± 14.6 cells/ml vs. 52.5 ± 42.6 cells/ml; p = 0.03). The increase in EPC correlated positively with LDL and total cholesterol/HDL ratio and negatively with peak oxygen consumption and oxygen consumption at anaerobic threshold, VEGF levels increased with exercise, with a strong trend toward significance (p = 0.055), nitric oxide (NO) levels remained unchanged [Citation32].
Regular exercise training augments the number of circulating EPCs in patients with cardiovascular risk factors and coronary artery disease and is associated with improved vascular function and NO synthesis, in this study, 20 patients with documented coronary artery disease and/or cardiovascular risk factors joined a 12-week supervised running training. Circulating EPCs defined by the surface markers CD34, KDR and CD133 were measured at baseline and after exercise training by flow cytometry, with a significant increase in circulating EPCs (2.9 ± 0.4-fold increase; p < 0.0001), which was positively correlated with both, the change of flow-mediated dilation (FMD) (r = 0.81, p < 0.001) and the increase of NO synthesis (r = 0.83, p < 0.001). Plasma VEGF and erythropoietin did not change in response to exercise. However, in this study, there was observed a positive correlation between the number of EPCs and erythropoietin at baseline (r = 0.70, p < 0.01) and after training (r = 0.73, p < 0.01) [Citation33].
Finally, higher habitual PhA level in patients with CAD was associated with higher flow-mediated dilation and EPC count. Nonetheless, FMD only significantly correlated with increased PhA level, but not EPC, suggesting that increased PhA improves endothelial function through mechanisms other than increasing EPC count [Citation34].
Strenuous activity in healthy individuals leads to a time-dependent increase in EPCs and MPE, which may be related to VEGF and IL-6. In a study, 18 healthy young men cycled for 4 h continuously at 70% of their individual anaerobic threshold. Peripheral blood was drawn at 16 predefined time points during and after finishing cycling. A significant rise in heart rate and leukocytes was obvious, whereas lactate levels and haematocrit did not change. The amount of circulating progenitor cells, mature endothelial cells and microparticles, quantified by flow cytometry, showed a significant time-dependent increase at 210/240 min. In addition, a very early rise in VEGF and later increase in IL-6, both measured by ELISA, were evident. All observed changes were normalised 24 h after finishing the test [Citation35].
The studies of Navasiolava et al. [Citation7] (cited in introduction) and Mobius-Winkler et al. [Citation35] remain the only studies that evaluated directly the serum concentrations of EMP during PhA.
In conclusion, this study shows that the standard protocol of aerobic exercise improves vascular and metabolic aspects of patients with arterial ED through improvement of endothelial function; other studies will examine the role of PhA in addition to medical therapy or its effects on hormonal parameters of these patients.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- O'Donovan G, Blazevich AJ, Boreham C, Cooper AR, Crank H, Ekelund U, Fox KR, Gately P, Giles-Corti B, Gill JMR, et al. The ABC of Physical Activity for Health: a consensus statement from the British Association of sport and exercise sciences. J Sports Sci 2010;28:573–591.
- Maio G, Saraeb S, Marchiori A. Physical activity and PDE5 inhibitors in the treatment of erectile dysfunction: results of a randomized controlled study. J Sex Med 2010;7:2201–2208.
- Janiszewski PM, Janssen I, Ross R. Abdominal obesity and physical inactivity are associated with erectile dysfunction independent of body mass index. J Sex Med 2009;6:1990–1998.
- Esposito K, Ciotola M, Giugliano F, Maiorino MI, Autorino R, De Sio M, Giugliano G, Nicoletti G, D'Andrea F, Giugliano D. Effects of intensive lifestyle changes on erectile dysfunction in men. J Sex Med 2009;6:243–250.
- Ribeiro F, Alves AJ, Duarte JA, Oliveira J. Is exercise training an effective therapy targeting endothelial dysfunction and vascular wall inflammation? Int J Cardiol 2010;141:214–221.
- Laufs U, Urhausen A, Werner N, Scharhag J, Heitz A, Kissner G, Böhm M, Kindermann W, Nickenig G. Running exercise of different duration and intensity: effect on endothelial progenitor cells in healthy subjects. Eur J Cardiovasc Prev Rehabil 2005;12:407–414.
- Navasiolava NM, Dignat-George F, Sabatier F, Larina IM, Demiot C, Fortrat JO, Gauquelin Koch G, Kozlovskaya IB, Custaud MA. Enforced physical inactivity increases endothelial microparticle levels in healthy volunteers. Am J Physiol Heart Circ Physiol 2010;299:H248–H256.
- García-Ortiz L, Recio-Rodríguez JI, Martín-Cantera C, Cabrejas-Sánchez A, Gómez-Arranz A, González-Viejo N, Iturregui-San Nicolás E, Patino-Alonso Maria C, Gómez-Marcos Manuel A; for the EVIDENT Group. Physical exercise, fitness and dietary pattern and their relationship with circadian blood pressure pattern, augmentation index and endothelial dysfunction biological markers: EVIDENT study protocol. BMC Publ Health 2010;10:233.
- Costa C, Virag R. The endothelial-erectile dysfunction connection: an essential update. J Sex Med 2009;6:2390–2404.
- Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 2003;107:1164–1169.
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–697.
- Papayannopoulou T. Current mechanistic scenarios in hematopoietic stem/progenitor cell mobilization. Blood 2004;103:1580–1585.
- Schatteman GC, Dunnwald M, Jiao C. Biology of bone marrow-derived endothelial cell precursors. Am J Physiol Heart Circ Physiol 2007;292:1–18.
- Güven H, Shepherd RM, Bach RG, Capoccia BJ, Link DC. The number of endothelial progenitor cell colonies in the blood is increased in patients with angiographically significant coronary artery disease. J Am Coll Cardiol 2006;48:1579–1587.
- Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human CD34+ AC133+ VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol 2007;35:1109–1118.
- Timmermans F, Van Hauwermeiren F, De Smedt M, Raedt R, Plasschaert F, De Buyzere ML, Gillebert TC, Plum J, Vandekerckhove B. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol 2007;27:1572–1579.
- Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol 1967;13:269–288.
- Shet AS. Characterizing blood microparticles: technical aspects and challenges. Vasc Health Risk Manag 2008;4:769–774.
- Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5 item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999;11:319–326.
- Benson CB, Aruny JE, Vickers MA Jr. Correlation of duplex sonography with arteriography in patients with erectile dysfunction. Am J Roentgenol 1993;160:71–73.
- Speel TG, van Langen H, Wijkstra H, Meuleman EJ. Penile duplex pharmaco-ultrasonography revisited: revalidation of the parameters of the cavernous arterial response. J Urol 2003;169: 216–220.
- Karvonen J, Vuorimaa T. Heart rate and exercise intensity during sports activities. Practical application. Sports Med 1988;5:303–311.
- Trichopoulou A. Traditional Mediterranean diet and longevity in the elderly: a review. Publ Health Nutr 2004;7:943–947.
- Seftel AD. Erectile dysfunction in the elderly: epidemiology, etiology and approaches to treatment. J Urol 2003;169:1999–2007.
- Galiano M, Pignot G, Costa C, Vallancien G, Virag R. Erectile dysfunction and cavernosal endothelial cells. Prog Urol 2010;20: 188–193.
- Camacho ME, Reyes-Ortiz CA. Sexual dysfunction in the elderly: age or disease? Int J Impot Res 2005;17 (Suppl 1):S52–S56.
- Hannan JL, Maio MT, Komolova M, Adams MA. Beneficial impact of exercise and obesity interventions on erectile function and its risk factors. J Sex Med. 2009;6 (Suppl 3):254–261.
- Kratzik CW, Lackner JE, Märk I, Rücklinger E, Schmidbauer J, Lunglmayr G, Schatzl G. How much physical activity is needed to maintain erectile function? Results of the Androx Vienna Municipality Study. How much physical activity is needed to maintain erectile function? Eur Urol 2009;55:509–516.
- Cheng JY, Ng EM. Body mass index, physical activity and erectile dysfunction: an U-shaped relationship from population-based study. Int J Obes (Lond) 2007;31:1571–1578.
- Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, D'Andrea F, D'Armiento M, Giugliano D. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA 2004;291:2978–2984.
- Adams V, Linke A, Breuckmann F, Leineweber K, Erbs S, Kränkel N, Bröcker-Preuss M, Woitek F, Erbel R, Heusch G, et al. Circulating progenitor cells decrease immediately after marathon race in advanced-age marathon runners. Eur J Cardiovasc Prev Rehabil 2008;15:602–607.
- Van Craenenbroeck EM, Vrints CJ, Haine SE, Vermeulen K, Goovaerts I, Van Tendeloo VF, Hoymans VY, Conraads VM. A maximal exercise bout increases the number of circulating CD34+/KDR+ endothelial progenitor cells in healthy subjects. Relation with lipid profile. J Appl Physiol 2008;104:1006–1013.
- Steiner S, Niessner A, Ziegler S, Richter B, Seidinger D, Pleiner J, Penka M, Wolzt M, Huber K, Wojta J, Minar E, Kopp CW. Endurance training increases the number of endothelial progenitor cells in patients with cardiovascular risk and coronary artery disease. Atherosclerosis 2005;181:305–310.
- Luk TH, Dai YL, Siu CW, Yiu KH, Chan HT, Fong DY, Lee SW, Li SW, Tam S, Lau CP, Tse HF. Habitual physical activity is associated with endothelial function and endothelial progenitor cells in patients with stable coronary artery disease. Eur J Cardiovasc Prev Rehabil 2009;16:464–471.
- Möbius-Winkler S, Hilberg T, Menzel K, Golla E, Burman A, Schuler G, Adams V. Time-dependent mobilization of circulating progenitor cells during strenuous exercise in healthy individuals. J Appl Physiol 2009;107:1943–1950.