Abstract
Objective. Blood endothelial progenitor cells (EPCs) and endothelial microparticles (EMPs) have been proposed as markers of endothelial dysfunction. Aim of this study was to evaluate an original immunophenotype of EPCs and EMPs in patients with isolated arterial erectile dysfunction (ED) and late onset hypogonadism (LOH) before and after androgen replacement therapy.
Materials and methods. Fifty patients (50–64 years) with ED and LOH were selected. EPC (CD45neg/CD34pos/CD144pos) and EMP (CD45neg/CD34neg/CD144pos) blood concentrations were evaluated by flow cytometry. Thirty patients received androgen replacement therapy (Tostrex® ProStrakan) for 6 months (group A), other 20 patients not received androgen therapy for the contraindications in their clinical history (group B).
Results. After 6 months, group B showed IIEF-5 score, peak systolic velocity and acceleration time significantly worse than group A; in addition EPCs and EMPs were significantly higher in group B compared to group A.
Conclusions. Patients with isolated arterial ED and LOH not treated with androgen therapy showed worst vascular parameters measured by penile Doppler and higher EPCs and EMPs compared to treated hypogonadal patients, hence, LOH appears to be an additional vascular risk factor, and these markers may be considered as predictors of cavernous artery disease. Finally, androgen therapy improves endothelial dysfunction.
Introduction
Cardiovascular disease (CVD) as coronary artery disease (CAD), cerebrovascular disease and peripheral artery disease (PAD) are the most important causes of morbidity and mortality in the developed and developing world. Particularly, CAD is the commonest cause of death worldwide. Testosterone (T) is an anabolic hormone with putative beneficial effects on men's health and restoration of normal T levels in deficient men represents an important key-point of male well-being. In the past years, it has emerged a possible linkage between androgen deficiency and CVD. Many studies noted that T deficiency might contribute to increased risk of CAD. Furthermore, adult's androgen deficiency defined that late onset hypogonadism (LOH) is frequently associated with increased levels of glucose, total cholesterol, low-density lipoprotein, increased production of pro-inflammatory cytokines and increased thickness of the arterial wall that all contribute to worsen endothelial function. The linkage between androgen deficiency and men's vascular health has a great impact in the modern approach to the ageing male, and should be further investigated to determine the therapeutic potential of androgens in men with vascular disease [Citation1].
All these conditions share an endothelial dysfunction which has a well established role in the pathogenesis of both atherosclerosis and plaque instability [Citation2,Citation3]. Hence, ED is more common in patients with overt or silent CAD and it is increasingly being regarded as an early clinical manifestation of a generalised CVD [Citation4–7]. Therefore, LOH may also favour the onset of ED [Citation8,Citation9].
Endothelial dysfunction may be evaluated in many ways and recently this has been done by estimating the number of circulating endothelial precursor cells (EPCs) and endothelial microparticles (EMPs). Hypotestosteronemia is associated with a low number of circulating PCs and EPCs in young hypogonadal subjects [Citation10], on the contrary, EPC positive for osteocalcin were significantly increased [Citation11]. EPCs are sub-populations of leucocytes that may differentiate into mature endothelial cells both in-vitro and in-vivo [Citation12]. The relevant contribution of these cells in the processes of re-endothelisation at sites of endothelial injury and neovascularisation has also been confirmed [Citation13–16]. Circulating EPC repair capability of the damaged endothelium suggests that these cells play a key role in maintaining the endothelial homeostasis. As a result, the number of EPCs may reflect ‘the vascular health’ of an individual and it is has been shown to be an independent predictor of CVD [Citation17]. The first EPC phenotype was defined by the presence of the following antigens in blood cells: CD34, CD133 and VEGFR2 (or KDR) [Citation13]. Many articles have reported statistically significant correlation between the number of this EPC subset in the blood with the presence of adverse cardiovascular outcomes. However, few studies have attempted to formally compare the functional properties of this EPC subset in hematopoietic and endothelial clonogenic assays to gain more insight in the biology of the phenotype used, namely to assess the identity of their clonal progeny which participates in vessel repair [Citation18,Citation19]. No clear evidence has been reported about the existence of specific markers which allows to prospectively isolate EPCs with a phenotype superior to the one so far described [Citation19]. Recently, some studies reported that purified CD34pos/CD133pos/KDRpos blood EPCs are highly enriched in hematopoietic progenitor cell (HPC) activity and express the universal hematopoietic cell-surface antigen CD45. Moreover, these cells are unable to give rise to endothelial colony-forming cells (ECFC) in-vitro. Indeed, CD45 is not expressed in endothelial cells even at the mRNA level. ECFC were enriched by 368-fold only when CD45neg/CD34pos cells were plated and not when CD45pos/CD34pos cells were used [Citation18,Citation20]. Therefore, the latter phenotype is a HPC-enriched cell subpopulation and thus EPCs are restricted to the phenotype CD45neg/CD34pos [Citation18]. A closer look at the biology of these EPCs indicate a progressive loss of CD133 and CD34 antigens and the expression of CD31, VE-cadherin (CD144) and Vwf, following their mobilisation into the general circulation [Citation21,Citation22]. In fact, CD34 is an adhesion molecule, expressed in haematopoietic stem cells, vascular progenitors and certain microvascular endothelium [Citation23]. CD144 is a single chain, transmembrane protein whose amino acid sequence indicates its homology with cadherins. It is specifically expressed in endothelial cells where it organises the adherent junctions which control permeability and migration and they are partially responsible to inhibit growth by contact [Citation24]. The concomitant presence of CD34 and CD144 antigens and the lost of the panleukocyte CD45 antigen assures that these cells are true stem/progenitor cells of endothelial nature [Citation17,Citation18,Citation20,Citation22]. Furthermore, CD144 or VEGFR-2 mRNA were not detected in CD45pos/CD34pos, but only in all sorted bone marrow CD34pos/CD45neg cells [Citation19]. Thus, the CD45neg/CD34pos/CD144pos phenotype defines a more endothelial committed cells [Citation18]. These EPCs have been shown able to form discrete colonies of endothelial cells in culture [Citation17]. On this account, we chose to evaluate this EPCs phenotype.
In addition to EPCs, microparticles of endothelial origin (EMPs) may be found in the general circulation. EMPs are membrane fragments which increase in several pathological conditions, including atherosclerosis, sepsis and diabetes mellitus [Citation25]. The main characteristics of these elements are the small dimension (<1.5 μm) and the externalisation of phosphatidylserine (PS). EMPs are regarded as markers of cardiovascular dysfunction with a pro-thrombotic role associated with risk factors such as those that LOH promotes [Citation26–30]. The percentage of EMPs is higher in patients with ED and inversely correlated with the IIEF-5 score [Citation31]. The presence of diabetes mellitus seems to be associated with a greater elevation of EMPs [Citation32]. Many studies have evaluated the correlation between EPCs or EMPs with the severity of CVD. Only few have assessed simultaneously these biomarkers and correlated them with the arterial status. There are no studies that have evaluated directly the serum concentration of EMP in hypogonadal patients.
On this basis, the present study was undertaken to evaluate the number of original immunophenotype both of EPCs and EMPs in patients with LOH and ED of isolated arterial origin before and after androgen replacement therapy. This clinical model seemed to us suitable to gain more insight into the balance between bone marrow endothelial repair mechanism and endothelial dysfunction in the manifestation of the clinical symptoms.
To accomplish this, 50 patients with isolated arterial dysfunctional-based ED and LOH, established by criteria of major scientific societies as International Society for the Study of the Aging Male (ISSAM), International Society of Andrology (ISA), European Association of Urology (EAU), European Accademy of Andrology (EAA) and American Society of Andrology (ASA) [Citation33], were selected and the number of circulating EPCs and EMPs (CD45neg/CD34neg/CD144pos) was determined. Within this group, 20 patients untreated for the presence of clinical contraindications, represented the controls.
Patients and methods
Patient selection
Fifty patients with arterial ED and LOH (group A) were enrolled, mean age: 59.3 ± 0.5 years (range: 50–64). The diagnosis of arterial ED was made when all the following criteria were fulfilled: (a) international index of erectile function (IIEF-5) score <21 [Citation34]; (b) cavernousal artery peak systolic velocity (PSV) <30 cm/sec, 10 and 20 min after the intracavernousal injection of alprostadil (20 μg) by echo colour Doppler [Citation35] and (c) acceleration time >110 msec [Citation36]. The diagnosis of LOH was made according to the criteria of major scientific societies as ISSAM, ISA, EAU, EAA and ASA [Citation33].
Exclusion criteria were the following: CAD, carotid and lower limbs atherosclerosis, smokers and diabetics.
By initial 50 patients, 30 patients underwent transdermal androgen replacement therapy (group A) for 6 months. Tostrex was administered with initial dose of 60 mg and subsequent adjustment of the dose after 14 days according to the serum concentrations of total testosterone.
Twenty patients were excluded from androgen treatment (group B) for the presence of clinical contraindications: abnormal digital rectal examination (n = 7), reported sleep apnea patients (n = 5), high hematocrit value (n = 4) and high values of transaminases n = 4.
All patients (group A and group B) underwent blood withdrawal (10 ml), for EPC and EMP measurements by flow cytometry. The protocol was approved by the internal Institutional Review Board and an informed written consent was obtained from each patient.
Blood EPC and EMP determination
EPCs and EMPs evaluation was performed in blood following incubation in erythrocyte lysing solution (Versalyse, IL, Milan, Italy) for 1 min. The suspension was then washed twice with phosphate buffer solution (PBS), centrifuged and the pellet was rapidly incubated in PBS containing the following monoclonal antibodies: anti-CD45 (10 μl), anti-CD34 (20 μl) and anti-CD144 (20 μl), respectively, labelled with r-phycoerythrin covalently bound to Texas red (ECD), fluorescein isothiocyanate (FITC) and r-phycoerythrin (PE), at room temperature for 20 min.
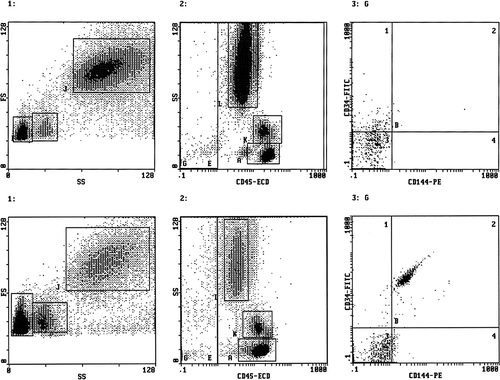
Each sample was analysed by flow cytometry (EPICS XL, Coulter Electronics, IL, Milan, Italy) using the following gating strategy (). Histograms 1 report the forward versus side scatter dot plot: three different cell populations can be identified; gate F: lymphocytes, gate I: monocytes, gate L: polymorphonuclear cells, Histograms 2 report the CD45pos (E) and CD45neg (G) cells; Histograms 3 report only the CD45neg cells (gate G) subdivided according to the staining with CD34 and CD144 antigens. EPCs were defined as CD45neg/CD34pos/CD144pos, whereas EMPs as CD45neg/CD34neg/CD144pos events. Fluocytometric analysis was conducted for 600 sec or 100,000 events (cells) whichever occurred first.
Figure 1. Representative fluocytometric scattergrams showing the gating strategy used in a normal healthy men (upper panels) and in a patient with late onset hypogonadism and erectile dysfunction (lower panels). Both histograms 1 report the forward versus side scatter dot plot: three different cell populations can be identified (gate F: lymphocytes; gate I: monocytes; gate J: polymorphonuclear cells). Both histograms 2 report the CD45pos (E) and CD45neg (G) cells. Both histograms 3 report only the CD45neg cells (gate G) subdivided according to the expression of CD34 and CD144 antigen staining. EPCs were defined as CD45neg/CD34pos/CD144pos, whereas EMP CD45neg/CD34neg/CD144pos.
Statistical analysis
Results are shown as mean ± SEM. EPC and EMP values were log-transformed before being analysed. Statistical analysis was conducted by one-way analysis of variance (ANOVA) followed by the Duncan Multiple range test or unpaired Student's t test, as appropriate. Correlation analysis was carried out by Pearson test. Statistical analysis was conducted using SPSS 10.0 for Windows. A p value lower than 0.05 was accepted as statistically significant.
Results
At baseline, no statistically significant difference was observed between the two groups, relatively to the following parameters: IIEF-5 score, serum testosterone concentration, peak systolic velocity, acceleration time and relatively to others vascular parameters as: end diastolic velocity, resistance index, intimal cavernous thickness ().
Table I. Penile dynamic echo-color doppler parameters, IIEF-5 score, T serum concentration and metabolic features (mean ± SEM) of the 50 patients (group A and group B) with arterial erectile dysfunction and late onset hypogonadism at baseline and after six months of transdermal androgen replacepent therapy for group A.
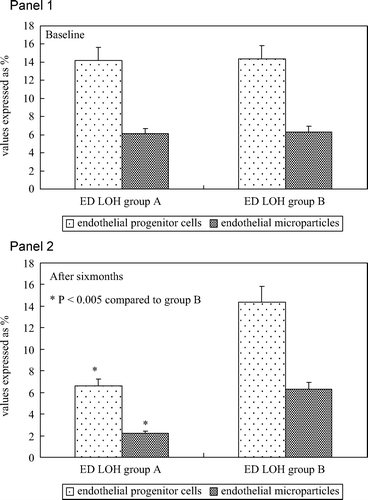
In addition, no statistically significant difference was observed between the two groups for the following metabolic characteristics: body mass index, total cholesterol, HDL, triglycerides, blood pressure detected during the enrolment visit (). At baseline, no statistically significant difference was observed between the two groups, relatively to serum EPCs (14.2 ± 1.2% vs 14.3 ± 2.3%) and EMP concentration (6.1 ± 0.4% vs 6.1 ± 1.1%) ().
Figure 2. Panel 1: Percentage of circulating endothelial progenitor cells (immunophenotype CD45neg/CD34pos/CD144pos) and percentage of circulating endothelial microparticles (immunophenotype CD45neg/CD34neg/CD144pos) in all patients with erectile dysfunction and late onset hypogonadism (group A and group B) at baseline. Panel 2: Percentage of circulating endothelial progenitor cells (immunophenotype CD45neg/CD34pos/CD144pos) and percentage of circulating endothelial microparticles (immunophenotype CD45neg/CD34neg/CD144pos) in all patients with erectile dysfunction and late onset hypogonadism (group A and group B) after transdermal androgen replacement therapy.
During the therapy, after 14 days, at first clinical control, no patient showed supraphysiologic levels of serum testosterone, therefore no patients changed the initial dose. No patient discontinued the study for the occurrence of complications (pathological digital rectal examination, high hematocrit values, sleep apnea, gynecomastia and increased liver enzymes).
After six months, group A showed mean serum T concentration significantly higher compared to baseline () and showed statistically significant difference relatively to all parameters investigated at baseline (IIEF-5 score, peak systolic velocity, accelleration time, body mass index, total cholesterol, HDL, triglycerides, blood pressure detected during 2nd visit), compared to group B ().
Finally, group A showed mean serum concentrations of EPCs (6.3 ± 1.2% vs 14.4 ± 2.8%) and EMP (2.2 ± 0.46% vs 6.2 ± 0.9%) significantly lower compared to group B ().
Discussion
This study evaluated the number of circulating original immunophenotype of EPCs and EMPs in patients with isolated arterial dysfunctional-based ED and late onset hypogonadism before and after replacement transdermal androgen therapy, since no data are available in these patients relative to these circulating phenotypes.
It is now well documented that the simultaneous measurement of both EPCs and EMPs may be proposed as biomarker of CVD and atherosclerotic complication and progression. We found that patients with arterial ED and LOH not treated with androgen therapy had worse penile vascular damage and metabolic features compared to treated patients and higher circulating original immunophenotype of EPCs and EMPs, suggesting that LOH improves endothelial dysfunction and androgen therapy improves reparative endothelial function, finally that these markers indicate a more severe vascular damage.
It is reported that low serum levels of T in aging men are associated with increased rate of mortality from CHD and other vascular events, and that T administration has beneficial effects on the pathophysiological markers and clinical symptoms of CHD. Testosterone improves the profile of the main risk factors involved in atherosclerosis such as obesity, the metabolic syndrome (MetS), type 2 diabetes and erectile dysfunction [Citation37].
T supplementation restores arterial vasoreactivity; reduces proinflammatory cytokines, total cholesterol and triglyceride levels; and improves endothelial function but also might reduce high-density lipoprotein levels [Citation38].
Foresta et al., evaluated 10 patients with hypogonadotropic hypogonadism treated with testosterone (gel 50 mg daily) for 6 months. This study showed a significant increase of EPCs after androgen treatment. In this study EPCs show, however, a different phenotype from ours, indeed, these cells are positive for CD133, CD34 and KDR, and their reduced baseline serum concentrations expresses an increased cardiovascular risk [Citation10].
The increase of these more endothelial regenerating EPCs may be able to rebuild the endothelial monolayer of the corpus cavernosum with amelioration of erectile function and protection from the disease progression in these patients. In patients with ED, the number of EPCs with the phenotype CD34pos/CD133pos/KDRpos is reduced and this reduction seems to be greater in presence of cardiovascular risk factors (cigarette smoke, hypertension, diabetes mellitus, obesity and dyslipidemia) [Citation6,Citation39,Citation40].
The apparent discrepancy with above mentioned studies may relate to the different immunophenotype evaluated. Indeed, more recently, an increased number of EPCs positive to osteocalcin, a marker of circulating osteogenic cells, has been reported in ED patients [Citation11]. In the attempt to understand the role played by EPCs on ED, the reduced number of CD34pos/CD133pos/KDRpos EPCs is an independent risk factor for ED in patients with known CAD. These findings suggest that EPCs may represent a link between cardiovascular risk factors, endothelial dysfunction and ED [Citation6]. It is clear now that there are distinct EPC sub-populations with different immunophenotype and biological properties [Citation17]. Cells progress from stem cell stage to the final mature cell of each single lineage, through successive progenitor cell stages [Citation41]. Because uniform criteria to identify these cells are lacking, we followed the proposal outlined by Case et al. They suggested to use the CD45neg/CD34pos phenotype because these cells form colonies of mature endothelial cells in a clonogenic in-vitro assay of endothelial cells [Citation18]. Therefore, probably these cells behave to a greater extent as true endothelial progenitors [Citation20]. Accordingly, CD45neg/CD34pos/CD144pos cells form endothelial cells in culture [Citation17]. The higher number of EPCs we found in patients with LOH, hence at increased CVD risks and corpus cavernosum atherosclerosis, are superimposible to previous evidences showing that distinct CVDs are associated with an increased circulating EPCs levels [Citation17,Citation42–44]. In CAD patients, several mechanistic possibilities have been advanced to explain the increase of EPCs. This activation may result from a variety of pro-inflammatory cytokines released in patients with coronary ischemia or after intravascular radiation [Citation15,Citation45]. Moreover, exercise-induced ischemia is associated with an increased number of circulating EPCs [Citation42,Citation44]. Patients with unstable angina have an increased number of EPCs compared with patients with stable angina, and the stabilisation of angina in these patients resulted in a two-fold decrease in circulating EPCs [Citation46]. EMPs (CD31pos/CD42neg and CD31pos/CD42pos) are elevated in ED patients with diabetes mellitus and independently involved in the pathogenesis of ED.
EMPs are released as consequence of endothelial dysfunction, atherogenesis and endothelial apoptotic processes. There are no studies that examine directly serum concentrations of EMP in hypogonadal men, or the effects of treatment with androgens on them. EMPs inversely correlate with the IIEF score in both diabetic and non-diabetic patients and multivariate analysis, corrected for confounding factors, showed that EMPs are the only independent predictor of the IIEF score [Citation31]. In addition, ED patients with or without diabetes mellitus have significantly higher EMPs (CD62pos) than non-diabetic men without ED. The EMPs (CD62pos)/EMPs (CD31pos) ratio, an index of endothelial activation (high ratio) or apoptosis (low ratio), was lowest in ED diabetic patients. These findings suggest that EMP increase in ED diabetic patients is consistent with an increased apoptotic activity [Citation32].
The results of this study conducted in patients with ED and LOH imply the same conclusion. These patients share with atherosclerotic patients a common picture of endothelial damage and markers of this dysfunction. This is sustained by the significant correlation between EPCs or EMPs, both bio-markers of endothelial dysfunction, with indices of ED severity (PSV, acceleration time and IMT).
In conclusion, this study showed that patients with isolated arterial ED and LOH not treated with androgen therapy showed worst vascular parameters measured by penile Doppler and higher EPCs and EMPs compared to treated hypogonadal patients with arterial ED, hence, LOH appears to be an additional vascular risk factor, and these markers may be considered predictors of cavernous artery disease. Finally, androgen therapy improves endothelial dysfunction of these patients.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Francomano D, Bruzziches R, Natali M, Aversa A, Spera G. Cardiovascular effect of testosterone replacement therapy in aging male. Acta Biomed 2010;81 (Suppl 1): 101–106.
- Widlansky ME, Gokce N, Keaney JF Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 2003;42:1149–1160.
- Sugiyama S, Kugiyama K, Aikawa M, Nakamura S, Ogawa H, Libby P. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler Thromb Vasc Biol 2004;24:1309–1314.
- Feldman HA, Johannes CB, Derby CA, Kleinman KP, Mohr BA, Araujo AB, McKinlay JB. Erectile dysfunction and coronary risk factors: prospective results from the Massachusetts male aging study. Prev Med 2000;30:328–338.
- Russel ST, Khandheria BK, Nehra A. Erectile dysfunction and cardiovascular disease. Mayo Clin Proc 2004;79:782–794.
- Baumhakel M, Werner N, Bohm M, Nickening G. Circulating endothelial progenitor cells correlate with erectile function in patients with coronary heart disease. Eur Heart J 2006;27:2184–2188.
- Gazzaruso C, Solerte SB, Pujia A, Coppola A, Vezzoli M, Salvucci F, Valenti C, Giustina A, Garzaniti A. Erectile dysfunction as a predictor of cardiovascular events and death in diabetic patients with angiographically proven asymptomatic coronary artery disease. A potential protective role for statins and 5-phosphodiesterase inhibitors. J Am Coll Cardiol 2008;51:2040–2044.
- Diemer T. Testosterone and erectile dysfunction. Urologe A 2010;49:26–31.
- Mikhail N. Does testosterone have a role in erectile function? Am J Med 2006;119:373–382.
- Foresta C, Caretta N, Lana A, De Toni L, Biagioli A, Ferlin A, Garolla A. Reduced number of circulating endothelial progenitor cells in hypogonadal men. J Clin Endocrinol Metab 2006;91:4599–4602.
- Foresta C, De Toni L, Biagioli A, Ganz F, Magagna S, Caretta N. Increased levels of osteocalcin-positive endothelial progenitor cells in patients affected by erectile dysfunction and cavernous atherosclerosis. J Sex Med 2009;7:751–757.
- Real C, Caiado F, Dias S. Endothelial progenitors in vascular repair and angiogenesis: how many are needed and what to do? Cardiovasc Hematol Disord Drug Targets 2008;8:185–193.
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–697.
- Werner N, Junk S, Laufs U, Link A, Walenta K, Bohm M, Nickenig G. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res 2003;93:e17–e24.
- Kong D, Melo LG, Gnecchi M, Zhang L, Mostoslavsky G, Liew CC, Pratt RE, Dzau VJ. Cytokine-induced mobilization of circulating endothelial progenitor cells enhances repair of injured arteries. Circulation 2004;110:2039–2046.
- Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 2004; 95:343–353.
- Güven H, Shepherd RM, Bach RG, Capoccia BJ, Link DC. The number of endothelial progenitor cell colonies in the blood is increased in patients with angiographically significant coronary artery disease. J Am Coll Cardiol 2006;48:1579–1587.
- Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human CD34+ AC133+ VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol 2007;35:1109–1118.
- Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 2008;28:1584–1595.
- Timmermans F, Van Hauwermeiren F, De Smedt M, Raedt R, Plasschaert F, De Buyzere ML, Gillebert TC, Plum J, Vandekerckhove B. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol 2007;27:1572–1579.
- Papayannopoulou T. Current mechanistic scenarios in hematopoietic stem/progenitor cell mobilization. Blood 2004;103:1580–1585.
- Schatteman GC, Dunnwald M, Jiao C. Biology of bone marrow-derived endothelial cell precursors. Am J Physiol Heart Circ Physiol 2007;292:1–18.
- Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, Baker MA, Greaves MF. Expression of the CD34 gene in vascular endothelial cells. Blood 1990;75:2417–2426.
- Ryu JK, Zhang LW, Jin HR, Piao S, Choi MJ, Tuvshintur B, Tumurbaatar M, Shin SH, Han JY, Kim WJ, Suh JK. Derangements in endothelial cell-to-cell junctions involved in the pathogenesis of hypercholesterolemia-induced erectile dysfunction. J Sex Med 2009;6:1893–1907.
- Shet AS. Characterizing blood microparticles: technical aspects and challenges. Vasc Health Risk Manag 2008;4:769–774.
- Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol 2009;5:673–681.
- Aversa A, Bruzziches R, Francomano D, Rosano G, Isidori AM, Lenzi A, Spera G. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med 2010;7:3495–3503. doi: 10.1111/j.1743-6109.2010.01931.x.
- Katabami T, Kato H, Asahina T, Hinohara S, Shin T, Kawata T, Ohta A, Iwamoto T, Tanaka Y. Serum free testosterone and metabolic syndrome in Japanese men. Endocr J 2010;57:533–539.
- Lunenfeld B. The relationship between sex hormones and the metabolic syndrome. Acta Biomed 2010;81 (Suppl 1): 79–84.
- Kintzel PE, Chase SL, Schultz LM, O'Rourke TJ. Increased risk of metabolic syndrome, diabetes mellitus, and cardiovascular disease in men receiving androgen deprivation therapy for prostate cancer. Pharmacotherapy 2008;28:1511–1522.
- Esposito K, Ciotola M, Giugliano F, Schisano B, Improta L, Improta MR, Beneduce F, Rispoli M, De Sio M, Giugliano D. Endothelial microparticles correlate with erectile dysfunction in diabetic men. Int J Impot Res 2007;19:161–166.
- Esposito K, Ciotola M, Giugliano F, Sardelli L, Giugliano F, Maiorino MI, Beneduce F, De Sio M, Giugliano D. Phenotypic assessment of endothelial microparticles in diabetic and nondiabetic men with erectile dysfunction. J Sex Med 2008;5:1436–1442.
- Wang C, Nieschlag E, Swerdloff RS, Behre H, Hellstrom WJ, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morales A, et al. ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male 2009;12:5–10.
- Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM. Development and evaluation of an abridged, 5 item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999;11:319–326.
- Benson CB, Aruny JE, Vickers MA Jr. Correlation of duplex sonography with arteriography in patients with erectile dysfunction. Am J Roentgenol 1993;160:71–73.
- Speel TG, van Langen H, Wijkstra H, Meuleman EJ. Penile duplex pharmaco-ultrasonography revisited: revalidation of the parameters of the cavernous arterial response. J Urol 2003;169:216–220.
- Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis 2009;207:318–327.
- Traish AM, Saad F, Feeley RJ, Guay A. The dark side of testosterone deficiency. III. Cardiovascular disease. J Androl 2009;30:477–494.
- Esposito K, Ciotola M, Maiorino MI, Giugliano F, Autorino R, De Sio M, Jannini E, Lenzi A, Giugliano D. Circulating CD34+ KDR+ endothelial progenitor cells correlate with erectile function and endothelial function in overweight men. J Sex Med 2009;6:107–114.
- Foresta C, Caretta N, Lana A, Cabrelle A, Palù G, Ferlin A. Circulating endothelial progenitor cells in subjects with erectile dysfunction. Int J Impot Res 2005;17:288–290.
- Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood 2005;106:1525–1531.
- Adams V, Lenk K, Linke A, Lenz D, Erbs S, Sandri M, Tarnok A, Gielen S, Emmrich F, Schuler G, Hambrecht R. Increase of circulating endothelial progenitor cells in patients with coronary artery disease after exercise-induced ischemia. Arterioscler Thromb Vasc Biol 2004;24:684–490.
- Massa M, Rosti V, Ferrario M, Campanelli R, Ramajoli I, Rosso R, De Ferrari GM, Ferlini M, Goffredo L, Bertoletti A, et al. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood 2005;105:199–206.
- Sandri M, Adams V, Gielen S, Linke A, Lenk K, Kränkel N, Lenz D, Erbs S, Scheinert D, Mohr FW, et al. Effects of exercise and ischemia on mobilization and functional activation of blood-derived progenitor cells in patients with ischemic syndromes: results of 3 randomized studies. Circulation 2005;111:3391–3399.
- Cho HJ, Kim HS, Lee MM, Kim DH, Yang HJ, Hur J, Hwang KK, Oh S, Choi YJ, Chae IH, et al. Mobilized endothelial progenitor cells by granulocyte-macrophage colony-stimulating factor accelerate reendothelization and reduce vascular inflammation after intravascular radiation. Circulation 2003;108:2918–2925.
- George J, Goldstein E, Abashidze S, Deutsch V, Shmilovich H, Finkelstein A, Herz I, Miller H, Keren G. Circulating endothelial progenitor cells in patients with unstable angina: association with systemic inflammation. Eur Heart J 2004;25:1003–1008.