Abstract
Objective. Altered circadian rhythms have been identified in untreated prostate cancer patients. Findings of restored rhythmicity following cancer treatment may have relevance for cancer control and symptom management. This study assessed and compared the cyclic patterns of hot flashes and activity levels in treated prostate cancer patients.
Methods. Data were collected during two 24-h periods among 47 prostate patients undergoing androgen deprivation therapy (ADT). Hot flashes were detected objectively through sternal skin conductance and by patients via electronic event marking. Activity levels were recorded on a wrist actigraphy device.
Results. The mean frequency of objectively measured and patient-reported hot flashes was 13.6 (SD = 14.3) and 12.6 (SD = 9.6), respectively. There were significant 24-h circadian rhythms of both hot flashes and activity levels. The peak of the rhythms occurred in early afternoon. There was no significant cross correlation between hot flashes and activity levels.
Conclusions. The acrophases of hot flashes and elevated activity levels in this study may represent a normalisation of circadian rhythms following ADT, pointing to the need for more research, including controlled, prospective chronobiologic studies. Future research may have important implications for the survival of prostate cancer patients and the identification of new and safe hot flash treatments.
Introduction
Virtually every hormone, neurotransmitter and physiologic process in the body follows a circadian rhythm. It is thought that circadian rhythms serve an adaptive function by allowing the body to synchronise internal functions with the external environment and to anticipate routine behaviours, such as the anticipatory secretion of digestive enzymes prior to regular mealtimes [Citation1]. It has further been proposed that reduced circadian rhythmicity is associated with less-optimal biological functioning, leading to negative health outcomes [Citation2]. In the context of prostate cancer, research has indicated that circadian rhythm profiles are altered in some men, raising the possibility that the disease is associated with altered rhythmicity.
Melatonin is used commonly as a marker of circadian rhythms because it is relatively unaffected by external cues other than light exposure. It is secreted primarily at night by the pineal gland. Across studies, serum melatonin concentrations were depressed in untreated patients with localised prostate cancer compared to control participants [Citation3,4] due to reduced pineal activity [Citation5,6] and did not show evidence of significant circadian rhythmicity. Disturbances in prolactin and thyroid stimulating hormone – but not luteinising hormone, total thyroxine, total testosterone and cortisol – have been found as well [Citation7]. Limited data indicate that prostatectomy has no effect on low-melatonin concentrations, but one case study suggests that depressed levels may normalise with androgen deprivation therapy (ADT) [Citation8].
Circadian rhythm alterations have been assessed in cancer populations by markers other than melatonin. Alternative markers include haematologic, skin temperature and activity cycles [Citation9]. Our group examined activity cycles in association with sleep for 7 days using a wrist actigraphy device among 60 men with prostate cancer on ADT [Citation10]. Night-time awakenings and daytime naps were indicated by changes in adjacent actigraphic epochs and cosinor analysis of the 7-day raw activity data found circadian rhythmicity for all participants with the mean peak of the rhythm (i.e. acrophase) occurring at 1422 h. Together, these studies provide evidence that circadian rhythms may be disrupted in patients with untreated prostate cancer but that ADT may restore function [Citation3–8,10].
Research among women suggests that hot flashes may be another marker of circadian rhythms. In one study, patient-reported hot flashes were shown to have a circadian rhythm among women with surgically induced menopause but not among women with a natural menopause [Citation11]. However, when hot flashes were measured objectively by sternal skin conductance among the latter group, a circadian rhythm of hot flashes was detected [Citation12]. The acrophase occurred at 1825 h in temporal proximity to the acrophase of core body temperature (TC), increases of which has triggered hot flashes in women [Citation12,13] and possibly in prostate cancer patients on ADT [Citation14]. Similarly, among treated breast cancer patients, a circadian rhythm of objectively measured hot flashes was found with an acrophase of 1610 h [Citation15].
Hot flashes are one of the key adverse events of ADT among prostate cancer patients, but no study to date has assessed if there is a cyclic rhythm to hot flashes in this population. Given the research above, we extended the assessment of circadian rhythms to hot flashes among prostate cancer patients on ADT and hypothesised that circadian patterns would be comparable to that of women since TC is inversely regulated by melatonin [Citation16]. Additionally, we assessed the relationship between hot flashes and activity levels since TC and activity levels have similar circadian patterns [Citation17] and acute episodes of activity can increase TC [Citation18]. Results may guide future research on circadian rhythms to improve cancer prevention and control by elucidating etiologic and diagnostic implications as well as aid research on symptom management.
Methods
Participants
Following approval from the University of Pennsylvania’s Institutional Review Board, 47 prostate cancer patients provided written informed consent and completed a cross-sectional quality-of-life study including ambulatory assessment of hot flashes [Citation19]. Eligibility criteria were ongoing ADT, Eastern Cooperative Oncology Group criteria of 0–3, and no current radiation, chemotherapy or myelosuppressive medications. Participants were recruited from local oncology and urology clinics, prostate cancer support groups and advertisements from June 2004 to February 2006.
Measures
Hot flashes were measured by both patient report and sternal skin conductance. The Biolog® monitor (UFI, Morro Bay, CA) was used to electronically record continuous skin conductance levels as well as at what time the men pressed the monitor’s event mark buttons to indicate a hot flash. Skin conductance levels were transmitted by lead wires from Meditrace® silver/silver chloride electrodes (Graphic Controls; Buffalo, NY) systematically attached to the sternum. Data were downloaded into a PC for evaluation by custom software (DPS v 3.3; UFI, Morro Bay, CA) and a trained data analyst (L.J.H.). A distinctive skin conductance profile consisting of a rapid increase of ≥1.78 micromhos within 45 s has been validated as an objective indication of a hot flash in prostate cancer patients [Citation20]. The co-occurrence of patient-reported and objective hot flashes was determined by an event mark immediately prior to or during the physiologic profile.
Wrist actigraphy (Actiwatch-64®; Mini Mitter Co., Inc., Bent, OR) was used to objectively monitor activity levels. This device uses a miniaturised omnidirectional accelerometer that integrates the degree and speed of motion at a maximum sampling rate of 32 Hz to continuously determine activity levels. The Actiwatch® has 64 KB of on-board memory and was programmed to record data in 0.25-min epochs. Actigraphy data were analysed by customised software (Actiware®-Sleep; Mini Mitter, Bent, OR), which uses algorithms to determine an activity count for each epoch.
Procedures
Research staff met in person with study participants for the collection of naturalistic data. Staff attached the Actiwatch® on participants’ non-dominant arm, activated the Biolog® system and obtained a list of participants’ current medications. Participants were instructed on how to event mark when having a hot flash and to go about their normal daily activities provided the Biolog® system did not get wet. After 24 h, research staff met again with participants to disconnect the Biolog® monitor. Another appointment was scheduled about 1 week later to obtain data for a total of 48 h on each participant. No adverse events with the devices were reported by participants. Medical records were reviewed to obtain clinical information. Participant compensation was $50.
Data analysis
Traditional cosinor analysis [Citation21] was used to fit both a 24-h and a 12-h rhythm to the data. For any statistically significant rhythm components, the acrophase (i.e. time of the peak of the rhythm), midline estimating statistic of rhythm (MESOR; i.e. a rhythm-adjusted mean) and the amplitude (i.e. mid distance between the peak and trough) were computed. Actigraphy was analysed as continuous data. Hot flash data, on the other hand, consisted of dichotomous ratings of a yes/no hot flash event with the majority of data categorised as an absence of hot flashes. Hot flash data were therefore aggregated across subjects into 1-h bins such that each hourly value is the sum of all hot flash events that occurred during that hour for all subjects. Circadian rhythms of hot flash events were analysed for both the objective profile and event marks. In addition, event marks lacking physiologic corroboration were analysed to assess whether the discordance between measures, particularly at night [Citation19], may be due to a circadian rhythm of sweat production [Citation22,23]. Next, the association between hot flashes and activity was examined by cross correlation between hot flash events by either measure or activity levels. For this analysis, actigraphy and hot flash data were aggregated into 5-min bins separately for each subject. Using Proc ARIMA in SAS, the cross correlations were computed with lags of up to 30 min (i.e. 6 bins) in either direction.
Results
Participants
The age of the 47 participants averaged at 71.9 (9.4) years and ranged from 54 to 88 years. Most of them were Caucasian (70.2%), married/partnered (80.9%), retired from work (68.1%) and lived with adults only (75.6%). A graduate school education was obtained by 42.6%, and 25.5% of the men earned an annual income of ≥$70,000. Over half of the men had received a diagnosis of prostate cancer 5 or more years prior, and about half of the men had been diagnosed with prostate cancer metastases by the time of study enrolment. Leuprolide acetate was the most common ADT taken by 42 men and 14 men were taking an antiandrogen, which was usually bicalutamide.
Hot flashes
No hot flashes were identified in one man by the objective profile, in two men by event marking and in one man by either measure. Among men who reported hot flashes, the mean frequency of event marks was 12.6 (SD = 9.6) and ranged from 1 to 33. Likewise, the mean frequency of objective hot flashes was 13.6 (SD = 14.3) and ranged from 1 to 63.
Circadian rhythms
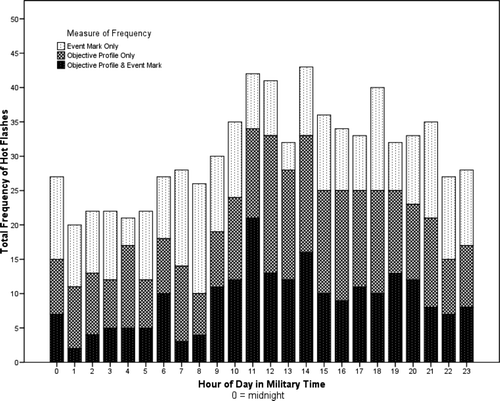
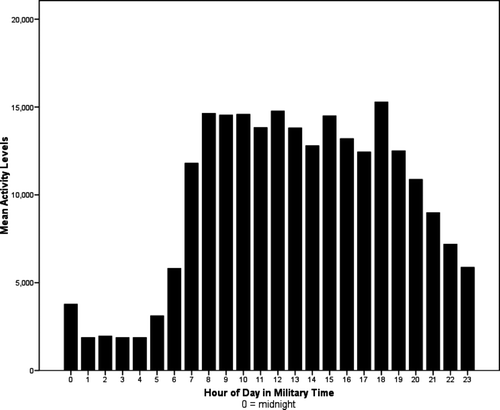
The aggregate hot flash data are shown in . Cosinor analyses confirmed a statistically significant 24-h rhythm in patient-reported [F(2,43) = 12.48, p < 0.0001] and objectively measured [F(2,43) = 43.22, p < 0.0001] hot flashes, but no evidence of 12-h ultradian rhythms (p > 0.05). Physiologically uncorroborated event marks did not have either 24- or 12-h significant cycles (p > 0.05). Activity levels, displayed in , had a statistically significant 24-h rhythm [F(2,571) = 1772.20, p < 0.0001] and the acrophases for both hot flashes and activity levels were closely clustered in the early afternoon. Results of the cosinor models are shown in . There were no statistically significant cross correlations between activity and hot flashes.
Table I. Circadian rhythm parameters.
Discussion
This research extends circadian rhythm analyses to treated prostate cancer patients. Significant findings included 24-h circadian rhythms, but not 12-h ultradian rhythms, for both hot flashes and activity levels. However, patient-reported hot flashes lacking physiologic corroboration did not occur in a significant circadian pattern as visually demonstrated in . Finally, no significant associations between hot flashes and activity levels were found suggesting that two factors are unrelated.
Hot flashes are purported to be caused by disturbances in the thermoregulatory system. Experimental and observational studies of women [Citation12,13] and a case report of a prostate cancer patient [Citation14] support that TC increases can trigger hot flashes. A thermal trigger of hot flashes in prostate cancer patients is further implicated by the current findings since hot flashes peaked in the afternoon when TC rises, as did activity levels, which have similar circadian pattern to TC [Citation17]. However, more research is needed to confirm if the natural peak in TC increases the likelihood to have hot flashes in prostate cancer patients since TC was not measured in this study.
The acrophase of hot flashes in men was earlier than in prior data in women and may be due to earlier TC phases in older men relative to middle-aged women [Citation24]. Different temporal peaks of circadian rhythms between hot flash populations are further supported by the similar acrophases between activity levels and hot flashes in this study. The similar acrophrases do not appear to be a result of a relationship between hot flashes and activity levels as no significant cross correlation was found. Instead, the similar acrophrases are likely due to common phase of entrainment to the body’s endogenous circadian clock.
Since the 1970s, a valid, objective measurement of hot flashes has been sought. Sternal skin conductance was demonstrated to perform better than other physiologic indicators in laboratory studies [Citation25], but in ambulatory studies, only modest concordance has been found between real time patient-reported hot flashes and hot flashes detected by sternal skin conductance [Citation19,26,27]. These results suggest that patients under-report hot flashes, but the lack of concordance may also be attributable to a circadian rhythm of sweating affecting skin conductance. However, we found no supporting evidence. Instead, uncorroborated patient-reported hot flashes may represent a particular subtype of hot flashes and not follow a circadian rhythm due to a triggering mechanism other than circadian-regulated TC increases. On the other hand, these subjective-only hot flashes may also be indicative of a reporting bias rather than a physiological phenomenon.
It is important to note that some men had none or very few hot flashes to assess circadian rhythms individually. It may be of interest to further investigate if the frequency of hot flashes in these patients is related to melatonin changes and survival. Among breast cancer patients taking tamoxifen, women reporting hot flashes were significantly less likely to have recurrent breast cancer [Citation28], and hence, hot flashes in breast cancer population may be a positive indicator of normal metabolism of tamoxifen by the cytochrome P450 2D6 enzyme (CYP2D6) [Citation29]. If similar results were found for prostate cancer patients on ADT, hot flashes could be used as a prognostic marker.
In conclusion, previous research found altered circadian rhythms in untreated prostate cancer patients [Citation3–7], but the current findings may represent a normalisation of circadian rhythms following ADT. These results need to be confirmed in prospective controlled studies with classic rhythm markers, such as melatonin and core body temperature and be compared to cancer tests measuring treatment response. There is evidence suggesting that melatonin has important etiologic and diagnostic implications, but the role of melatonin in malignant growth and in detecting early cancer, cancer remission and cancer recurrence with intra-individual melatonin changes needs further research [Citation30]. Melatonin may also affect hot flashes via its regulation of core body temperature. More research on the thermoregulatory mechanisms of hot flashes is needed to identify new treatments since current treatments among cancer patients are limited by side effects, contraindications or limited efficacy [Citation31,32].
Acknowledgement
The authors thank Nalaka Gooneratne, MD, for his contributions to this study.
Declaration of interest: The authors report no conflicts of interest. This work was supported by the Department of Defense [grant number DAMD17-02-1-0125]. The content of this publication does not necessarily reflect the views or policies of the Department of Defense.
References
- Panda S, Hogenesch JB. It’s all in the timing: many clocks, many outputs. J Biol Rhythms 2004;19(5):374–387.
- Maury E, Ramsey KM, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res 2010;106(3):447–462.
- Bartsch C, Bartsch H, Flüchter SH, Harzmann R, Bichler KH, Gupta D. Serum melatonin and cortisol rhythms of patients with benign and malignant tumors of the prostate. Neuro Endocrinol Lett 1982:4(3):176.
- Bartsch C, Bartsch H, Flüchter SH, Harzmann R, Attanasio A, Bichler KH, Gupta D. Circadian rhythms of serum melatonin, prolactin, and growth hormone in patients with benign and malignant tumors of the prostate and in non-tumor controls. Neuroendocrinol Lett 1983;5(6):377–386.
- Bartsch C, Bartsch H, Flüchter SH, Attanasio A, Gupta D. Evidence for modulation of melatonin secretion in men with benign and malignant tumors of the prostate: relationship with pituitary hormones. J Pineal Res 1985;2(2)121–132.
- Bartsch C, Bartsch H, Schmidt A, Ilg S, Bichler KH, Flüchter SH. Melatonin and 6-sulfatoxymelatonin circadian rhythms in serum and urine of primary prostate cancer patients: evidence for reduced pineal activity and relevance of urinary determinations. Clin Chim Acta 1992;209(3):153–167.
- Bartsch C, Bartsch H. Prostate cancer and tumor stage dependent circadian neuroendocrine disturbances. Aging Male 1998;1(3):188–199.
- Bartsch C, Bartsch H, Flüchter SH, Attanasio A, Gupta D. Melatonin rhythms in prostate-cancer patients – effect of operation and hormone-treatment. J Neural Transm 1986;21:491.
- Mormont MC, Lévi F. Circadian-system alterations during cancer processes: a review. Int J Cancer 1997;70(5):241–247.
- Hanisch LJ, Gooneratne NS, Soin K, Gehrman PR, Vaughn DJ, Coyne JC. Sleep and daily functioning during androgen deprivation therapy for prostate cancer. Eur J Cancer Care 2010. DOI: 10.1111/j.136523542010.01226.x
- Albright DL, Voda AM, Smolensky MH, His B, Decker M. Circadian rhythms in hot flashes in natural and surgically-induced menopause. Chronobiol Int 1989;6(3):279–284.
- Freedman RR, Norton D, Woodward S, Cornélissen G. Core body temperature and circadian rhythm of hot flashes in menopausal women. J Clin Endocrinol Metab 1995;80(8):2354–2358.
- Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol 1999;181(1):66–70.
- Hanisch LJ, Mao JJ, Gehrman PR, Vaughn DJ, Coyne JC. Brief report: increases in core body temperature precede hot flashes in a prostate cancer patient. Psychooncology 2009; 18(5):564–567.
- Carpenter JS, Gilchrist JM, Chen K, Gautam S, Freedman RR. Hot flashes, core body temperature, and metabolic parameters in breast cancer survivors. Menopause 2004;11(4):375–381.
- Cagnacci A, Elliott JA, Yen SS. Melatonin: a major regulator of the circadian rhythm of core temperature in humans. J Clin Endocrinol Metab 1992;75(2):447–452.
- Aschoff J. Circadian rhythms in man. Science 1965;148(3676):1427–1432.
- Fujishima K. Thermoregulatory responses during exercise and a hot water immersion and the affective responses to peripheral thermal stimuli. Int J Biometeorol 1986;30(1):1–19.
- Hanisch LH, Palmer SC, Marcus SC, Hantsoo L, Vaughn DJ, Coyne JC. Comparison of objective and patient-reported hot flash measures in men with prostate cancer. J Support Oncol 2009;7(4):1–5.
- Hanisch LJ, Palmer SC, Donahue A, Coyne JC. Validation of sternal skin conductance for detection of hot flashes in prostate cancer survivors. Psychophysiology 2007;44(2):189–193.
- Halberg F, Tong YL, Johnson EA. Circadian system phase – an aspect of temporal morphology; procedures and illustrative examples. In: von Myersbach H, editor.The cellular aspects of biorhythms. New York: Springer-Verlag; 1968. pp. 20–48.
- Aoki K, Kondo N, Shibasaki M, Takano S, Tominaga H, Katsuura T. Circadian variation of sweating responses to passive heat stress. Acta Physiol Scand 1997;161(3):397–402.
- Timbal J, Colin J, Boutelier C. Circadian variations in the sweating mechanism. J Appl Physiol 1975;39(2):226–230.
- Monk TH, Buysse DJ, Reynolds CF III, Kupfer DJ, Houck PR. Circadian temperature rhythms of older people. Exp Gerontol 1995;30(5):455–474.
- Freedman RR. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology 1989;26(5):573–579.
- Carpenter JS, Andrykowski MA, Freedman RR, Munn R. Feasibility and psychometrics of an ambulatory hot flash monitoring device. Menopause 1999;6(3):209–215.
- Thurston RC, Blumenthal JA, Babyak MA, Sherwood A. Emotional antecedents of hot flashes during daily life. Psychosom Med 2005;67(1):137–146.
- Mortimer JE, Flatt SW, Parker BA, Gold EB, Wasserman L, Natarajan L, Pierce JP; WHEL Study Group. Tamoxifen, hot flashes and recurrence in breast cancer. Breast Cancer Res Treat 2008;108(3):421–426.
- Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL, Flockhart DA, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol 2005;23(36):9312–9318.
- Bartsch C, Bartsch H. Pineal Gland and Cancer – An Epigenetic Approach to the Control of Malignancy: Evaluation of the Role of Melatonin. (Madame Curie Bioscience Database [Internet]). 2000.
- Carpenter JS. State of the science: hot flashes and cancer. Part 2: management and future directions. Oncol Nurs Forum 2005;32(5):969–978.
- Rada G, Capurro D, Pantoja T, Corbalán J, Moreno G, Letelier LM, Vera C. Non-hormonal interventions for hot flushes in women with a history of breast cancer. Cochrane Database Syst Rev 2010;8(9):CD004923.