Abstract
In aging men, serum endogenous testosterone is inversely associated with common carotid intima-media thickness (IMT) and directly with beneficial plasma lipid levels; however, the relationship to endothelial function is poorly characterized. We examined the association between serum testosterone and endothelium-dependent brachial artery flow-mediated dilatation (FMD) in middle-aged to elderly men. A group of 83 men aged 40–69 years (mean 55.9 ± 7.5 [SD]) with andropausal symptoms were studied. We measured their serum lipids, testosterone, luteinizing hormone, mean carotid IMT and brachial artery FMD by high resolution B-mode ultrasound. Brachial FMD correlated inversely with vessel diameter (r = −0.38, p = 0.0004), alcohol consumption (r = −0.22, p = 0.047) and serum testosterone (r = −0.27, p = 0.01), but not with luteinizing hormone. In multivariate analysis, FMD was explained by testosterone (β = −0.17, p = 0.0226), high density lipoprotein cholesterol (β = 4.17, p = 0.0312) and vessel diameter (β = −4.37, p < 0.0001) when adjusted for age, body mass index, triglycerides, blood pressure, carotid IMT, smoking, alcohol consumption, cardiovascular diseases and use of lipid lowering medication (HMG-CoA reductase inhibitors). In middle-aged to elderly men, there is an inverse correlation between serum testosterone and brachial FMD. These data suggest that testosterone may have an adverse effect on systemic endothelial function.
Introduction
Atherosclerosis is a chronic process with a characteristic sex difference resulting in an earlier and more extensive cardiovascular disease in men compared to women [Citation1]. This difference has been suggested to be partly sex hormone-induced. Disturbances in vascular function occur in atherosclerosis. Vascular function is mediated through endothelium and the underlying smooth muscle cells and is commonly characterized as the vessel ability to vasodilatate to a standard stimulus. Vascular endothelial and smooth muscle functions can be assessed non-invasively with ultrasound by measuring flow- and nitrate-mediated dilatations (FMD, NMD), respectively, in the brachial artery. Normal endothelial function maintains the intravascular homeostasis by regulating arterial tone, platelet and leukocyte interactions, coagulation, fibrinolysis and vascular growth [Citation2]. In contrast, endothelial dysfunction, reflected by decreased FMD in brachial arteries, correlates with coronary endothelial function, the extent of coronary atherosclerosis [Citation3] and predicts cardiovascular events [Citation4,Citation5]. Impaired NMD responses have also been reported in subjects with atherosclerotic risk factors [Citation6] and in patients with coronary artery disease [Citation7].
Both estrogens and androgens are recognized to exert specific effects on vascular function through their receptors expressed in a variety of cells of the vascular wall [Citation8,Citation9]. Endogenous testosterone has been associated both with increased [Citation10] and impaired [Citation11] FMD responses in men. Similarly, the response to physiological levels of androgen supplementation in men has given contradictory results by both impairing [Citation12,Citation13] and enhancing vasodilatation, [Citation14,Citation15] and the latter has been observed also in women [Citation16]. In both human and animal experiments, androgen administration induces rapid vasodilatation in arterial beds, e.g., coronaries and aorta with varying degree of sensitivity [Citation17,Citation18]. In men, this seems to sustain in a long-term follow-up [Citation14]. However, adverse vascular effects related to androgens have also been reported. Supraphysiological levels of exogenous androgens in the form of anabolic steroids are suggested to cause dose-dependent impairment of vascular function accompanied by an atherogenic shift in the lipid profile (low high-density lipoprotein [HDL] cholesterol, high triglycerides), platelet aggregation, activation of the coagulation cascade [Citation19,Citation20].
Thus, the results concerning the association of testosterone and vascular function are controversial, and the vascular effects of declining testosterone levels in aging men are poorly understood. In the Turku Male Aging Study, we have previously shown a beneficial effect of endogenous testosterone on carotid artery intima-media thickness (IMT) [Citation21] and on serum lipid profile [Citation22] in middle-aged and older men. We hypothesized that men with symptoms of andropause, low testosterone increased IMT and adverse lipid profile would have impaired FMD all together. In the present study, we have examined the relations between endogenous serum testosterone and luteinizing hormone with vascular function in the same population.
Materials and methods
The study individuals
The study population was derived from the Turku Male Aging Study, which originally consisted of all volunteer men (N = 15.496) aged 40–69 years from the city of Turku, Finland. We approached a randomly selected group of 99 who exceeded the threshold for andropausal symptoms, i.e., positive symptoms of late-onset hypogonadism. Of the 99 men contacted, 83 (84%) volunteered for further examinations. The symptoms of late-onset hypogonadism were evaluated by the Turku 3 Question-query and the aging male symptoms’ (AMS) scale [Citation23]. The Turku 3 Question-query had three questions: During the last 5 years, have you had (1) weakening in tasks requiring strength; (2) decreased libido; or (3) depression? Each “yes” answer yielded one point with a consequent range of scores from 0–3. A sum score ≥2 was considered high and suggestive of late-onset hypogonadism and all study subjects fulfilled this condition. The AMS had 17 questions on symptoms with a scale from 1 to 5: no symptoms (1 point), mild (2 points), moderate (3 points), severe (4 points) and to very severe symptoms (5 points). A sum score >36 was considered positive and indicative of late-onset hypogonadism [Citation23]. The issues of general health [Citation24] were determined by addressing smoking, alcohol consumption and medical background. The medical background was determined by diseases diagnosed by the individual’s physician. Subjects were asked whether they had been diagnosed with diabetes mellitus or cardiovascular disease, which included coronary artery disease and hypertension. None of the study subjects had been diagnosed with diabetes mellitus. The study individuals were asked about their prescription medications and cardiovascular disease medication was considered to include antihypertensives and medication for coronary artery disease. HMG CoA (3-hydroxy-3-methyl-glutaryl-Coentzyme A) reductase inhibitors were inquired separately. Current smoking status was assessed during the ultrasound examination. Alcohol consumption was estimated by reported drinking frequency (1 = no alcohol use, 2 = once a year or less, 3 = 3–4 times a year, 4 = once in 2 months, 5 = 2–3 times a month, 6 = once a week, 7 = twice a week, 8 = daily or almost daily). Body mass index (BMI) was determined by square of height (m2)/weight (kg) based on height and weight information provided by the subjects.
The Joint Ethics Committee of the University of Turku and the University of Turku Central Hospital approved the study. The study subjects were informed about the study in a covering letter and returning the questionnaire was considered as acceptance to participate in the study. A written consent was obtained from each individual before the blood samples were drawn and ultrasound studies were carried out. The participants had the right to discontinue their participation at any time. The study was conducted according to the Declaration of Helsinki.
Ultrasound studies
All studies were performed by an experienced vascular sonographer who was blinded to the clinical characteristics of the participants, using an Acuson Sequoia 512 mainframe (Acuson, Mountain View, CA) with a 13.0-MHz linear-array transducer. The studies were performed after an overnight fast between 07:30 and 10:30 hours. To minimize disturbing stimuli, all studies were carried out in a silent clinical research laboratory room. Blood pressure was measured twice during the ultrasound examination from the nondominant arm using a sphygmomanometer. All studies were done following a predetermined, standardized scanning protocol [Citation21,Citation25] for the right and left carotid arteries and left brachial artery. All scans were digitally stored for subsequent off-line analysis, which was done blinded for the individuals’ clinical details.
Brachial FMD
The diameter of the left brachial artery was measured at rest and during reactive hyperemia. Increased flow was induced by using a pneumatic tourniquet placed around the forearm inflated up to 250 mmHg for 4.5 minutes before release. A fixed distance from an anatomic marker three diameters of the artery at end-diastole was measured at base line and then 40, 60, 80, 100 and 120 seconds after cuff release. The maximum FMD was determined by the average value of the measurements at each time point and expressed as percentage compared to the baseline. Nitrate-mediated, endothelium-independent vasodilatation was tested by scanning the artery 4 minutes after a sublingual dose of 1.25 mg isosorbide dinitrate.
Carotid IMT
The proximal part of the carotid bulb was identified, and the segment of the common carotid artery far wall 1 cm proximal to the bulb was scanned. Two angles were used in each case: anterior oblique and lateral. Two end-diastolic frames of the best image quality were selected and analyzed for maximum IMT from the carotid far wall with a minimum of four measurements. The average readings from these frames were calculated.
Laboratory measurements
Venous fasting (at least 10 hours) blood samples were drawn from an antecubital vein in the morning (07:30–11:30 hours). Serum testosterone was measured using coated-tube radioimmunoassay (Spectria, Orion Diagnostica, Finland). Serum luteinizing hormone was measured using time-resolved fluoroimmunometric assay (AutoDelfia, Perkin Elmer-Wallac, Finland). Serum total cholesterol, HDL-cholesterol and triglyceride concentrations were measured using standard enzymatic methods with Boehringer Mannheim GmbH (Mannheim, Germany) reagents, with an automated analyzer (Hitachi 917; Hitachi Ltd., Tokyo, Japan). Low-density lipoprotein (LDL) cholesterol concentration was calculated using the Friedewald’s equation [Citation26]. LDL-cholesterol was not determined in 18 men, because they had serum triglyceride levels greater than 4.0 mmol/l.
Statistical analysis
The mean values are expressed as mean ± standard deviation (SD). Triglycerides were log transformed due to skewness of distribution. Bivariate correlates between the study variables were analyzed by calculating Spearman’s correlation coefficients. Multivariate analysis was performed by linear regression analyses. All statistical analyses were performed using Statistical Analysis System (SAS Institute, Gary, NC).
Results
The characteristics of the men are shown in . Sixteen men (19%) had been diagnosed with cardiovascular disease, and 13 men (16%) reported use of cardiovascular disease medications. HMG-CoA reductase inhibitors were used by 5 men (6%).
Table I. Characteristics (mean and SD, or prevalence) of 83 aging men in the Turku Male Aging Study.
The bivariate correlations between FMD, NMD and testosterone with other essential study variables are displayed in the . Serum testosterone correlated inversely with FMD, BMI, triglycerides and IMT, and directly with serum luteinizing hormone and HDL-cholesterol. FMD correlated inversely with the vessel diameter and alcohol consumption and directly with NMD. NMD correlated inversely with age, BMI, IMT and alcohol consumption. Luteinizing hormone was not significantly associated with FMD or NMD.
Figure 1. Univariate correlates for flow-mediated dilatation (FMD), nitrate-mediated dilatation (NMD) and testosterone. Correlation coefficients are shown from the variables statistically significant (p < 0.05). BMI, body mass index; T, testosterone; HDL, HDL-cholesterol; LDL, LDL-cholesterol; TG, triglycerides; IMT, intima-media thickness; BD, brachial diameter; AC, alcohol consumption.
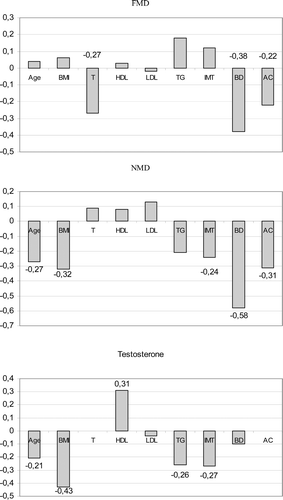
In multivariate analyses, the independent correlates of FMD and NMD were assessed by taking into account: age, BMI, testosterone, total cholesterol, HDL-cholesterol, triglycerides, blood pressure, carotid IMT, brachial artery diameter, smoking, alcohol consumption, cardiovascular disease and HMG-CoA reductase inhibitor medication. In a multivariate model for FMD, an inverse association was detected between testosterone and FMD (β = –0.17, p = 0.0226) and a direct association between FMD and HDL-cholesterol (β = 4.17, p = 0.0312, ). In the multivariate model for NMD, the only significant correlate was vessel diameter (β = −7.3, p < 0.0001).
Table II. Multivariate models of the relations between risk variables and brachial artery flow-mediated dilatation in 83 aging men in the Turku Male Aging Study.
Discussion
Suggesting possible beneficial role of androgens in preventing cardiovascular disease, we have previously shown that endogenous serum testosterone concentration in aging men is related with thinner carotid IMT [Citation21] as well as lower triglycerides and higher HDL-cholesterol levels [Citation22]. In the present study, we found, however, an inverse relation between endogenous testosterone and endothelial function in aging men that remained statistically significant in a multivariate analysis. Previous observations on the relations between testosterone and endothelial function are mixed. In older men, testosterone replacement in physiological concentrations seems to increase endothelium dependent dilatation [Citation14]. Webb et al. recently demonstrated that oral testosterone treatment modestly improved myocardial perfusion in older men with low plasma testosterone and coronary heart disease but had no effect on arterial endothelial function assessed by arterial tonometry and salbutamol technique. In younger men, most but not all studies [Citation27] have reported adverse influences of testosterone replacement on vascular function [Citation12,Citation13].
The hormonal mechanisms involved behind the direct effects on vascular function can be divided to nongenomic and genomic [Citation28], where the first is characterized by rapid ion-channel alterations and the latter represents the classic steroid receptor, e.g., androgen receptor mediated effect. These hormone-induced mechanisms influence simultaneously on vascular function [Citation16] and can not be clearly distinguished from one another in vivo.
Estrogens and androgens seem to target the endothelium through their specific receptors in a variety of cells in the vascular wall [Citation8,Citation9]. Activation of both of these specific receptors appear to induce vasodilatation by releasing nitric oxide [Citation8,Citation29,Citation30], and reports of the vasodilatation caused by estrogens in both sexes are established [Citation8,Citation31]. Testosterone–induced, nitrate-mediated vasodilatation [Citation30,Citation32] and preservation of thinner carotid IMT [Citation33] have all been argued to be mediated by general and/or local (endothelial) aromatase activity resulting in testosterone’s conversion to estrogens [Citation34]. According to our previous studies, aromatization seems not to be the mechanism at least on lipid profile [Citation22] and on carotid IMT [Citation21]. However, in macrophages, testosterone inhibits nitric oxide synthase enzyme resulting in decreased nitric oxide levels [Citation35], in part implicating an atherogenic character of testosterone.
In contrast to the classic receptor mediated effect, the nongenomic actions induced by testosterone are independent of androgen receptor action [Citation36,Citation37]. By these methods, androgens seem to improve endothelial function [Citation37] and the rapid vasodilatation is mediated by blocking ion channels, e.g., membrane calcium influx [Citation36]. Moreover, testosterone’s nongenomic effects seem to induce vasodilatation by several mechanisms [Citation28], and it has been suggested that due to these (and possibly other) nongenomic actions, testosterone might protect from cardiovascular disease [Citation28].
Our study has several limitations. The study included a relatively limited number of subjects. Nevertheless, the findings were rather distinct and the small sample size is unlikely to detract the validity of the main findings. The men studied were selected based on high symptom score associated with hypogonadism, and may therefore not be representative of the average male population. However, there are no indications that the measured symptoms might cause a significant selection bias. The measured symptoms of late-onset hypogonadism seem to be only loosely associated with serum total testosterone levels [Citation22], suggesting a minor importance of these symptoms in screening for hypogonadal men. In addition, our study was cross-sectional, so these associations do not necessarily demonstrate causal relations between testosterone and FMD. The dietary data and exercise habits were not collected, and it is acknowledged that this information could, in part, elucidate the observed associations. In this study, the use of HMG-CoA reductase inhibitors was successfully adjusted, in effort to control their both direct and indirect effects on vascular function [Citation38].
Several studies in aging men have shown inverse association between testosterone and cardiovascular disease [Citation39]. The treatment of aging men with testosterone supplementation is increasing [Citation40], despite that the effects on cardiovascular health are not entirely understood. Testosterone may have several beneficial effects, such as improved insulin sensitivity, fatty acid metabolism and observed thinner common carotid IMT [Citation21,Citation22,Citation41]. However, our current results dispute the possibility that individuals with impaired sex-hormone status might benefit from testosterone replacement therapy to decelerate the progression of atherosclerosis and protect from its clinical sequelae, e.g., chronic heart disease and ischemic stroke. To improve the prevention and therapy of cardiovascular diseases, there is a need to better understand the mechanisms and role of hormones, especially the role of endogenous testosterone in these processes. The current findings prompt further research to elucidate in more detail the association between androgens, FMD and the development of atherosclerosis.
Declaration of interest: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. This work was supported by The Academy of Finland (grant no 210888 and 201283), Emil Aaltonen Foundation and The Research foundation of Orion Corporation.
References
- Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation 1994;90:583–612.
- Celermajer DS. Endothelial dysfunction: Does it matter? Is it reversible? J Am Coll Cardiol 1997;30:325–333.
- Neunteufl T, Katzenschlager R, Hassan A, Klaar U, Schwarzacher S, Glogar D, Bauer P, Weidinger F. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis 1997;129:111–118.
- Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation 2007;115:2390–2397.
- Chan SY, Mancini GB, Kuramoto L, Schulzer M, Frohlich J, Ignaszewski A. The prognostic importance of endothelial dysfunction and carotid atheroma burden in patients with coronary artery disease. J Am Coll Cardiol 2003;42:1037–1043.
- Adams MR, Robinson J, McCredie R, Seale JP, Sorensen KE, Deanfield JE, Celermajer DS. Smooth muscle dysfunction occurs independently of impaired endothelium-dependent dilation in adults at risk of atherosclerosis. J Am Coll Cardiol 1998;32:123–127.
- Raitakari OT, Seale JP, Celermajer DS. Impaired vascular responses to nitroglycerin in subjects with coronary atherosclerosis. Am J Cardiol 2001;87:217–9, A8.
- Losordo DW, Kearney M, Kim EA, Jekanowski J, Isner JM. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation 1994;89:1501–1510.
- Fujimoto R, Morimoto I, Morita E, Sugimoto H, Ito Y, Eto S. Androgen receptors, 5 alpha-reductase activity and androgen-dependent proliferation of vascular smooth muscle cells. J Steroid Biochem Mol Biol 1994;50:169–174.
- Karakitsos D, Patrianakos AP, De Groot E, Boletis J, Karabinis A, Kyriazis J, Samonis G, et al. Androgen deficiency and endothelial dysfunction in men with end-stage kidney disease receiving maintenance hemodialysis. Am J Nephrol 2006;26:536–543.
- Herman SM, Robinson JT, McCredie RJ, Adams MR, Boyer MJ, Celermajer DS. Androgen deprivation is associated with enhanced endothelium-dependent dilatation in adult men. Arterioscler Thromb Vasc Biol 1997;17:2004–2009.
- Zitzmann M, Brune M, Nieschlag E. Vascular reactivity in hypogonadal men is reduced by androgen substitution. J Clin Endocrinol Metab 2002;87:5030–5037.
- Sader MA, Griffiths KA, Skilton MR, Wishart SM, Handelsman DJ, Celermajer DS. Physiological testosterone replacement and arterial endothelial function in men. Clin Endocrinol (Oxf) 2003;59:62–67.
- Kang SM, Jang Y, Kim JY, Chung N, Cho SY, Chae JS, Lee JH. Effect of oral administration of testosterone on brachial arterial vasoreactivity in men with coronary artery disease. Am J Cardiol 2002;89:862–864.
- Webb CM, McNeill JG, Hayward CS, de Zeigler D, Collins P. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation 1999;100:1690–1696.
- Worboys S, Kotsopoulos D, Teede H, McGrath B, Davis SR. Evidence that parenteral testosterone therapy may improve endothelium-dependent and -independent vasodilation in postmenopausal women already receiving estrogen. J Clin Endocrinol Metab 2001;86:158–161.
- Rosano GM, Leonardo F, Pagnotta P, Pelliccia F, Panina G, Cerquetani E, della Monica PL, et al. Acute anti-ischemic effect of testosterone in men with coronary artery disease. Circulation 1999;99:1666–1670.
- Yue P, Chatterjee K, Beale C, Poole-Wilson PA, Collins P. Testosterone relaxes rabbit coronary arteries and aorta. Circulation 1995;91:1154–1160.
- Ebenbichler CF, Sturm W, Gänzer H, Bodner J, Mangweth B, Ritsch A, Sandhofer A, et al. Flow-mediated, endothelium-dependent vasodilatation is impaired in male body builders taking anabolic-androgenic steroids. Atherosclerosis 2001;158:483–490.
- Sullivan ML, Martinez CM, Gennis P, Gallagher EJ. The cardiac toxicity of anabolic steroids. Prog Cardiovasc Dis 1998;41:1–15.
- Mäkinen J, Järvisalo MJ, Pöllänen P, Perheentupa A, Irjala K, Koskenvuo M, Mäkinen J, et al. Increased carotid atherosclerosis in andropausal middle-aged men. J Am Coll Cardiol 2005;45:1603–1608.
- Mäkinen JI, Perheentupa A, Irjala K, Pöllänen P, Mäkinen J, Huhtaniemi I, Raitakari OT. Endogenous testosterone and serum lipids in middle-aged men. Atherosclerosis 2008;197:688–693.
- Heinemann LAJ, Zimmermann T, Vermeulen A, Thiel C. A New “Aging Male’s Symptoms” (AMS) Rating Scale. Aging Male 1999;2:105–114.
- Koivumaa-Honkanen H, Honkanen R, Viinamäki H, Heikkilä K, Kaprio J, Koskenvuo M. Self-reported life satisfaction and 20-year mortality in healthy Finnish adults. Am J Epidemiol 2000;152:983–991.
- Juonala M, Viikari JS, Laitinen T, Marniemi J, Helenius H, Rönnemaa T, Raitakari OT. Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: The cardiovascular risk in young Finns study. Circulation 2004;110:2918–2923.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502.
- Akishita M, Hashimoto M, Ohike Y, Ogawa S, Iijima K, Eto M, Ouchi Y. Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res 2007;30:1029–1034.
- Yildiz O, Seyrek M. Vasodilating mechanisms of testosterone. Exp Clin Endocrinol Diabetes 2007;115:1–6.
- Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: Relevance to endothelial dysfunction in hypercholesterolemia. Circ Res 2001;88:145–151.
- Costarella CE, Stallone JN, Rutecki GW, Whittier FC. Testosterone causes direct relaxation of rat thoracic aorta. J Pharmacol Exp Ther 1996;277:34–39.
- Sader MA, McCredie RJ, Griffiths KA, Wishart SM, Handelsman DJ, Celermajer DS. Oestradiol improves arterial endothelial function in healthy men receiving testosterone. Clin Endocrinol (Oxf) 2001;54:175–181.
- Lew R, Komesaroff P, Williams M, Dawood T, Sudhir K. Endogenous estrogens influence endothelial function in young men. Circ Res 2003;93:1127–1133.
- Nathan L, Shi W, Dinh H, Mukherjee TK, Wang X, Lusis AJ, Chaudhuri G. Testosterone inhibits early atherogenesis by conversion to estradiol: Critical role of aromatase. Proc Natl Acad Sci USA 2001;98:3589–3593.
- Diano S, Horvath TL, Mor G, Register T, Adams M, Harada N, Naftolin F. Aromatase and estrogen receptor immunoreactivity in the coronary arteries of monkeys and human subjects. Menopause 1999;6:21–28.
- Friedl R, Brunner M, Moeslinger T, Spieckermann PG. Testosterone inhibits expression of inducible nitric oxide synthase in murine macrophages. Life Sci 2000;68:417–429.
- English KM, Jones RD, Jones TH, Morice AH, Channer KS. Testosterone acts as a coronary vasodilator by a calcium antagonistic action. J Endocrinol Invest 2002;25:455–458.
- Williams MR, Dawood T, Ling S, Dai A, Lew R, Myles K, Funder JW, et al. Dehydroepiandrosterone increases endothelial cell proliferation in vitro and improves endothelial function in vivo by mechanisms independent of androgen and estrogen receptors. J Clin Endocrinol Metab 2004;89:4708–4715.
- Sakabe K, Fukuda N, Wakayama K, Nada T, Shinohara H, Tamura Y. Lipid-altering changes and pleiotropic effects of atorvastatin in patients with hypercholesterolemia. Am J Cardiol 2004;94:497–500.
- English KM, Mandour O, Steeds RP, Diver MJ, Jones TH, Channer KS. Men with coronary artery disease have lower levels of androgens than men with normal coronary angiograms. Eur Heart J 2000;21:890–894.
- Cunningham GR. Testosterone replacement therapy for late-onset hypogonadism. Nat Clin Pract Urol 2006;3:260–267.
- Pitteloud N, Mootha VK, Dwyer AA, Hardin M, Lee H, Eriksson KF, Tripathy D, et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care 2005;28:1636–1642.