Abstract
Objective: The study sought to clarify the relationship between sex hormone levels and lower urinary tract symptoms (LUTS) in patients with benign prostate hyperplasia. Methods: Between 2007 and 2010, serum total testosterone (TT), free testosterone, and estradiol were prospectively measured in patients who were transferred to our university hospital. The 924 subjects were divided into two groups. Group I (n = 646) were treated with an alpha blocker only and group II (n = 278) were treated with an alpha blocker + a 5-alpha reductase inhibitor over 3 months before their visit. Clinical conditions were assessed by digital rectal examination, prostate-specific antigen, International Prostate Symptom Score (IPSS), transrectal ultrasonography and maximum urinary flow rate and postvoid residual urine. Results: The mean age was 69.65 ± 6.56 years. The total IPSS and subscore (storage symptom) was significantly associated with age (p < 0.001/p < 0.05) and the TT level (p < 0.05/p < 0.05). TT level was significantly decreased in patients with ≥4 episodes of nocturia. The TT level was significantly related to the presence of severe LUTS (p < 0.05). Conclusions: Endogenous testosterone may have a beneficial effect on lower urinary tract function and that a high frequency of nocturia may induce testosterone deficiency.
Introduction
Benign prostate hyperplasia (BPH) is very common among aging men and can cause lower urinary tract symptoms (LUTS), which can be bothersome and tend to diminish the quality of life [Citation1–3]. The prevalence of LUTS increases from 8% in the fourth decade of life to >70% in the seventh decade of life, and BPH is the most common cause of LUTS in middle-aged and elderly men [Citation3].
Two factors thought to play a role in the etiogenesis of BPH are aging and androgens [Citation3–6]. Androgens are important for prostate development and growth, in addition to the maintenance of structural and functional integrity [Citation4–6]. As sex steroid hormones are an important determinant of prostate growth, it is likely that these hormones also contribute to the development and maintenance of LUTS secondary to BPH in older men [Citation5,Citation6].
While the serum testosterone level steadily decreases after 40-years-of-age, it has been demonstrated that 5-alpha reductase (5AR) activity and androgen receptor levels increase with aging [Citation7,Citation8]. Thus, aging prostate cells gradually become more sensitive to dihydrotestosterone (DHT), which in turn stimulates cell replication in the prostate. However, LUTS can also occur in aging men without BPH, and the severity of LUTS secondary to BPH is not necessarily correlated with prostate volume [Citation9]. Recently, testosterone deficiency has been the focus of research because it is a possible risk factor for metabolic syndrome, and preliminary evidence indicates that men with LUTS benefit from treatment with testosterone [Citation10].
Psychological and physical stresses such as deprivation of food and sleep significantly decrease testosterone levels [Citation11]. Nocturia is the most bothersome symptom and cause of sleep deprivation in patients with BPH, and stressors such as frequent awakening can also decrease the testosterone serum level. The aim of the present study was to determine the possible association between blood hormone levels and the severity of LUTS, especially with respect to the frequency of nocturia in men.
Methods
In all, 924 men who were transferred to the university hospital to receive high quality treatment between June 2007 and June 2010 were recruited for this study. The subjects had been treated with an alpha blocker or an alpha blocker + a 5-alpha reductase inhibitor (5ARI) over 3 months. The exclusion criteria for the current study were age <50 years, presence of an endocrine disease as primary hypogonadism and hyperprolactinoma, presence of a prostate disease treated with antiandrogen therapy (except 5ARI for the treatment of BPH), current treatment for cerebrovascular diseases, and presence of psychological diseases.
We prospectively divided the study population into a group treated with an alpha blocker only (group I, n = 646) and a group treated with an alpha blocker + 5ARI (group II, n = 278) for >3 months in private practice before they visited our hospital. No men that received 5ARI only were enrolled.
BPH was assessed by digital rectal examination (DRE), serum prostate-specific antigen (PSA) level determination, International Prostate Symptom Score (IPSS), transrectal ultrasonography (B&K Medical, Herlev, Denmark), and maximum urinary flow rate (Qmax) based on uroflowmetry and postvoid residual urine (PVR) by bladder ultrasonography. Patients with abnormal DRE findings or an elevated serum PSA level (>4.0 ng/ml) underwent prostate biopsies to exclude the possibility of prostate cancer. The strategy of prostate biopsy was systemic 12-core biopsy [Citation12,Citation13]. Five hundred twenty eight of the 924 patients underwent prostate biopsies to rule out prostate cancer. Upon establishing the definitive absence of prostate cancer, these patients were enrolled. According to the data from our institution, the cancer detection rate ranged from 24.8%–25.9% [Citation14].
We measured the serum concentrations of total testosterone (TT), free testosterone (FT), and estradiol (E2) by radioimmunoassay. The IPSS, determined using a validated eight-item questionnaire, was used to assess LUTS. The severity of symptoms was classified as mild (≤7), moderate (8–19), or severe (≥20). Storage symptom scores were evaluated by summing responses to IPSS questions 2 (frequency), 4 (urgency) and 7 (nocturia). In order to evaluate the voiding frequency, we used IPSS in the patients with ≤5 episodes of nocturia and the record of number of nocturnal voiding in the patients with ≥6 episodes of nocturia.
Voiding symptoms scores were evaluated by summing responses to IPSS questions 3 (intermittency), 5 (weak stream) and 6 (straining). The correlations between the different variables were first estimated using Pearson’s correlation coefficients. Statistical significance was defined as p < 0.05. The Pearson’s rank correlation test was first used to determine the relationship between the IPSS and serum sex hormone levels. The strongly significantly p < 0.01) associated factors with IPSS by Pearson’s rank correlation test were estimated using multivariate linear regression models.
Results
The final study population consisted of 924 men with a mean age of 69.65 ± 6.56 years (range, 50–86 years). The mean IPSS of the study population was 18.01 ± 8.88 (1–35), the mean prostate volume was 57.58 ± 20.13 cm3 (range, 18–108 cm3), and the mean Qmax and PVR were 10.7 ± 4.86 ml/s (range, 2.3–28.7 ml/s) and 102.80 ± 143.02 ml, respectively. The mean values for PSA, TT, FT, and E2 were 6.47 ± 8.52 ng/ml (range, 0.22–59.8 ng/ml), 4.56 ± 1.70 ng/ml (range, 1.1–10.9 ng/ml), 8.54 ± 3.02 pg/ml (range, 1.15–18.8 pg/ml) and 17.73 ± 7.87 pg/ml (range, 4.6–43.0 pg/ml), respectively. The serum TT levels in group 2 (4.88 ± 1.71) was higher than group 1 (4.24 ± 1.54). Endocrine variables and clinical characteristics of the study population are shown in .
Table I. Patient characteristics.
When we divided the study group based on alpha blocker and 5ARI treatment, groups I and II comprised 646 and 278 men, respectively.
Based on statistical analysis, IPSS correlated positively with age (r = 0.425, p < 0.001) and negatively with the TT level (r = −0.153, p < 0.05), but IPSS did not correlate with the serum FT and E2 levels, prostate volume and Qmax. The TT level was also negatively correlated with IPSS storage symptom scores (r = −0.147, p < 0.05), but not with voiding symptom scores. The mean value for Qmax in groups I and II were 9.86 ± 3.73 and 9.67 ± 4.04, respectively. The mean values for PVR in groups I and II were 114.18 ± 89.12 and 143.02 ± 102.80 ml, respectively.
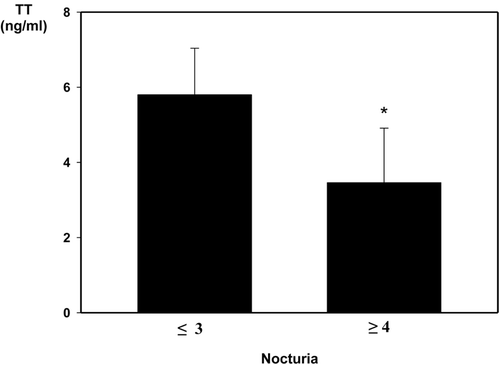
The nocturia subscore was negatively correlated with the TT level. The results were similar between the two groups, with the exception of the nocturia domain (). In addition, we divided the nocturia subscore into ≤3 times and ≥4 times according to the severity. The mean level of TT by nocturia subscore was 5.86 ± 1.24 ng/ml (range, 3.6–10.9 ng/ml) and 3.46 ± 1.45 ng/ml (range, 1.1–8.6 ng/ml), respectively (). We analyzed the relationship of age and the level of TT (). Among the three groups based on the severity of LUTS, we evaluated the median level of TT in each group (4.98 ng/ml, 4.52 ng/ml and 4.21 ng/ml, respectively). An increased risk of LUTS was seen in men with a lower serum concentration of TT, although the mean level of testosterone was in the normal range.
Table II. Correlations of total IPSS and subscores with hormone levels (alpha blocker only/alpha blocker + 5ARI).
The prostate volume was positively correlated with age (r = 0.303, p < 0.001) and PSA level (r = 0.651, p < 0.001), but not with serum levels of the sex hormones, IPSS, and Qmax. Age was positively correlated with prostate volume (r = 0.049, p < 0.001), IPSS (r = 0.163, p = 0.037) and PSA level (r = 0.501, p < 0.001), and negatively with the FT level (r = −0.382, p < 0.001; ). With multivariate linear regression-derived ß coefficients and p values, age and TT were significantly associated with IPSS. There was no correlation with age and the TT level or age and the other sex hormone levels with Pearson correlation analysis. There was a significant association between transitional zone (TZ) volume and TT/E2 ratio, but no correlation between TZ volume and E2 levels ().
Table III. Pearson correlations of hormonal variables with age, prostate volume, IPSS and Qmax.
Table IV. Pearson correlations of hormonal variables (E2 and TT/E2 ratio) with prostate volume (total and transitional zone).
Univariate analysis revealed that cardiovascular disease (CVD), diabetes mellitus (DM) and hypertension (HTN) were positively correlated with the prevalence of nocturia ().
Table V. Analysis of risk factors for nocturia using clinical parameters.
No significant differences existed among mild, moderate and severe LUTS cases with respect to mean age, prostate volume and PSA level. The FT and E2 levels were similar in patients with mild, moderate and severe LUTS, but the TT level was significantly lower in patients with higher IPSS total scores (p < 0.05; ).
Table VI. Clinical and laboratory characteristics of patients according to IPSS severity.
Discussion
LUTS are common in older men, although intercultural and ethnic differences in the prevalence of LUTS and BPH have been reported [Citation15–17]. An analysis of the data from the Third National Health and Nutrition Examination Survey reported that 22% of men aged 70–79 years in the United States had undergone noncancerous prostate surgery, presumably for LUTS induced by BPH, and that the prevalence of specific urinary symptoms increases with age [Citation18]. Furthermore, several large-scale epidemiologic studies have identified age as the most important risk factor for BPH and LUTS [Citation19–21]. These data were also confirmed in the present study.
In general, the patients with symptomatic BPH received two kinds of prolonged medical therapy: alpha blocker only or alpha blocker + 5ARI. The effects of alpha blocker or/and 5ARI differ. Alpha blocker decreases the tone in prostate smooth muscle and partial detrussor muscle, and 5ARI decreases the volume of the prostate. Considering these different modes of action, the patients were divided into two groups.
Androgens are well-accepted as the second risk factor for BPH. Sex hormones are an important determinant of prostate growth, and they also contribute to the development and maintenance of LUTS secondary to BPH in older men [Citation22,Citation23].
Psychic stress or sleep deprivation significantly decreases testosterone levels [Citation24,Citation25]. Currently, we do not know which of the following occurs first: nocturia-induced stress decreases testosterone levels, or age induces decreased testosterone levels, which causes nocturia. In the prostate, testosterone is converted into the more potent androgen, DHT, by a type II 5AR. It is thought that DHT has a central role in the development and maintenance of BPH because the inhibition of 5AR activity is associated with decreased serum DHT concentrations and decreased prostate size [Citation26].
It has been hypothesized that E2 potentiates the effects of androgens in inducing BPH by stimulating the androgen receptor [Citation7,Citation27]. Despite the co-localization of androgen receptors with estrogen receptors in the lower urinary tract, few studies have been conducted on the relationship between LUTS and sex hormones status [Citation28].
Several studies have focused on the relationship between serum androgen concentrations and clinical BPH in elderly men, but the results have not been consistent. Joseph et al. [Citation29] reported that a large prostate volume is marginally associated with an increased TT level in African-American men, but Meikle et al. [Citation30] found an inverse correlation between prostate volume and TT level in 214 Caucasian male twins. Others have not found a significant association between the TT level and prostate volume [Citation4,Citation20,Citation31]. No consistent correlation was observed between the TT and calculated FT levels and LUTS in another study, but a relationship was seen between androstenediol glucuronide, a metabolite of DHT, and E2[Citation32]. Additionally, some studies have reported that prostate volume was not associated with the FT level or bioavailable testosterone level after adjustment for age [Citation2,Citation4]. In our results, the TT/E2 ratio, but not E2, was related with TZ volume. However, the association between the prostate volume and sex hormones is contentious, although estrogen plays a crucial role in the pathogenesis of BPH through the estrogen receptor [Citation33]. The present results may reflect the inclusion of patients treated with 5ARI.
In the present study, the dominant predictor of LUTS was patient age. However, in older men with symptomatic BPH, a significant association was observed between the severity of LUTS and the serum TT level (total IPSS, storage symptom scores). CVD, diabetes DM and HTN, and the medications to treat them, were positively affected to LUTS. However those subjects with induced nocturia also could experience decreased production of testosterone. Further studies with large numbers of subjects are needed for conclusive resolution.
Presently, significantly negative relationships between the TT level and IPSS total score and storage symptom scores were evident on univariate and multivariate analyses. These findings suggest that endogenous testosterone contributes to the development of LUTS.
The effect of testosterone on LUTS might be explained by the effects of testosterone on alpha1-adrenergic receptors, phosphodiesterase type 5 activity, and Rho-kinase activation/endothelin activity, all of which are androgen-dependent [Citation34]. No significant association between IPSS and other serum sex hormones levels was observed. The prostate volume did not correlate with the severity of LUTS, serum testosterone levels, or other sex hormone levels. Only age was a significant predisposing factor for prostate volume based on statistical analysis. Taken together, the age-related growth of the prostate cannot be explained by a mere increase or decrease in serum androgens. Schultheiss et al. [Citation35] reviewed previous studies and concluded that the link between androgens and age-related growth of the prostate might be explained by a shift of the hormonal ratio (e.g. the androgen-to-estrogen ratio), the changing intraprostatic hormonal level or a modified action of hormones and respective receptors, as well as of intraprostatic enzymes (e.g. 5AR).
Conclusions
In patients with ≥4 episodes of nocturia, testosterone levels were significantly lower than in patients with ≤3 episodes of nocturia, although many factors can induce LUTS. This study supports the notion that endogenous testosterone has a beneficial effect on lower urinary tract function and suggests that frequent nocturia may induce testosterone deficiency.
Acknowledgements
This study was sponsored by Chonbuk National University in 2009. Dong Gon Kim is a co-corresponding author. M. K. Kim, MD, PhD received grants from Chonbuk National University. However, Chonbuk National University only had a financial role in this research.
Declaration of Interest: The authors report no conflict of interest.
References
- Chute CG, Panser LA, Girman CJ, Oesterling JE, Guess HA, Jacobsen SJ, Lieber MM. The prevalence of prostatism: a population-based survey of urinary symptoms. J Urol 1993;150:85–89.
- Liu CC, Wang CJ, Huang SP, Chou YH, Wu WJ, Huang CH. Relationships between American Urological Association symptom index, prostate volume, and disease-specific quality of life question in patients with benign prostatic hyperplasia. Kaohsiung J Med Sci 2004;20:273–278.
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol 1984;132:474–479.
- Partin AW, Oesterling JE, Epstein JI, Horton R, Walsh PC. Influence of age and endocrine factors on the volume of benign prostatic hyperplasia. J Urol 1991;145:405–409.
- Montie JE, Pienta KJ. Review of the role of androgenic hormones in the epidemiology of benign prostatic hyperplasia and prostate cancer. Urology 1994;43:892–899.
- Griffiths K, Eaton CL, Harper ME, Peeling B, Davies P. Steroid hormones and the pathogenesis of benign prostatic hyperplasia. Eur Urol 1991;20 Suppl 1:68–77.
- Bruchovsky N, Rennie PS, Batzold FH, Goldenberg SL, Fletcher T, McLoughlin MG. Kinetic parameters of 5 alpha-reductase activity in stroma and epithelium of normal, hyperplastic, and carcinomatous human prostates. J Clin Endocrinol Metab 1988;67:806–816.
- Barrack ER, Bujnovszky P, Walsh PC. Subcellular distribution of androgen receptors in human normal, benign hyperplastic, and malignant prostatic tissues: characterization of nuclear salt-resistant receptors. Cancer Res 1983;43:1107–1116.
- Barry MJ, Cockett AT, Holtgrewe HL, McConnell JD, Sihelnik SA, Winfield HN. Relationship of symptoms of prostatism to commonly used physiological and anatomical measures of the severity of benign prostatic hyperplasia. J Urol 1993;150:351–358.
- Amano T, Imao T, Takemae K, Iwamoto T, Nakanome M. Testosterone replacement therapy by testosterone ointment relieves lower urinary tract symptoms in late onset hypogonadism patients. Aging Male 2010;13:242–246.
- Wu JL, Wu RS, Yang JG, Huang CC, Chen KB, Fang KH, Tsai HD. Effects of sleep deprivation on serum testosterone concentrations in the rat. Neurosci Lett 2011;494:124–129.
- Hodge KK, McNeal JE, Terris MK, Stamey TA. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol 1989;142:71–4; discussion 74.
- Presti JCJr, O’Dowd GJ, Miller MC, Mattu R, Veltri RW. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J Urol 2003;169:125–129.
- Choi KS, Park JK. Epidemiologic characteristics of prostate cancer detection. Korean J Urol 2009;50:1054–1058.
- Kang D, Andriole GL, Van De Vooren RC, Crawford D, Chia D, Urban DA, Reding D, et al. Risk behaviours and benign prostatic hyperplasia. BJU Int 2004;93:1241–1245.
- Jin B, Turner L, Zhou Z, Zhou EL, Handelsman DJ. Ethnicity and migration as determinants of human prostate size. J Clin Endocrinol Metab 1999;84:3613–3619.
- Li MK, Garcia LA, Rosen R. Lower urinary tract symptoms and male sexual dysfunction in Asia: a survey of ageing men from five Asian countries. BJU Int 2005;96:1339–1354.
- Platz EA, Smit E, Curhan GC, Nyberg LM, Giovannucci E. Prevalence of and racial/ethnic variation in lower urinary tract symptoms and noncancer prostate surgery in U.S. men. Urology 2002;59:877–883.
- Garraway WM, Collins GN, Lee RJ. High prevalence of benign prostatic hypertrophy in the community. Lancet 1991;338:469–471.
- Schatzl G, Brössner C, Schmid S, Kugler W, Roehrich M, Treu T, Szalay A, et al. Endocrine status in elderly men with lower urinary tract symptoms: correlation of age, hormonal status, and lower urinary tract function. The Prostate Study Group of the Austrian Society of Urology. Urology 2000;55:397–402.
- Madersbacher S, Haidinger G, Temml C, Schmidbauer CP. Prevalence of lower urinary tract symptoms in Austria as assessed by an open survey of 2,096 men. Eur Urol 1998;34:136–141.
- Sciarra F, Toscano V. Role of estrogens in human benign prostatic hyperplasia. Arch Androl 2000;44:213–220.
- Andriole G, Bruchovsky N, Chung LW, Matsumoto AM, Rittmaster R, Roehrborn C, Russell D, Tindall D. Dihydrotestosterone and the prostate: the scientific rationale for 5alpha-reductase inhibitors in the treatment of benign prostatic hyperplasia. J Urol 2004;172:1399–1403.
- Andersen ML, Tufik S. The effects of testosterone on sleep and sleep-disordered breathing in men: its bidirectional interaction with erectile function. Sleep Med Rev 2008;12:365–379.
- Leproult R, Van Cauter E. Effect of 1 week of sleep restriction on testosterone levels in young healthy men. JAMA 2011;305:2173–2174.
- McConnell JD, Bruskewitz R, Walsh P, Andriole G, Lieber M, Holtgrewe HL, Albertsen P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med 1998;338:557–563.
- Yeh S, Miyamoto H, Shima H, Chang C. From estrogen to androgen receptor: a new pathway for sex hormones in prostate. Proc Natl Acad Sci USA 1998;95:5527–5532.
- Liu CC, Huang SP, Li WM, Wang CJ, Chou YH, Li CC, Huang CH, Wu WJ. Relationship between serum testosterone and measures of benign prostatic hyperplasia in aging men. Urology 2007;70:677–680.
- Joseph MA, Wei JT, Harlow SD, Cooney KA, Dunn RL, Jaffe CA, Montie JE, Schottenfeld D. Relationship of serum sex-steroid hormones and prostate volume in African American men. Prostate 2002;53:322–329.
- Meikle AW, Stephenson RA, Lewis CM, Middleton RG. Effects of age and sex hormones on transition and peripheral zone volumes of prostate and benign prostatic hyperplasia in twins. J Clin Endocrinol Metab 1997;82:571–575.
- Roberts RO, Jacobson DJ, Rhodes T, Klee GG, Leiber MM, Jacobsen SJ. Serum sex hormones and measures of benign prostatic hyperplasia. Prostate 2004;61:124–131.
- Rohrmann S, Nelson WG, Rifai N, Kanarek N, Basaria S, Tsilidis KK, Smit E, et al. Serum sex steroid hormones and lower urinary tract symptoms in Third National Health and Nutrition Examination Survey (NHANES III). Urology 2007;69:708–713.
- Tsurusaki T, Aoki D, Kanetake H, Inoue S, Muramatsu M, Hishikawa Y, Koji T. Zone-dependent expression of estrogen receptors alpha and beta in human benign prostatic hyperplasia. J Clin Endocrinol Metab 2003;88:1333–1340.
- Koritsiadis G, Stravodimos K, Mitropoulos D, Doumanis G, Fokitis I, Koritsiadis S, Constantinides C. Androgens and bladder outlet obstruction: a correlation with pressure-flow variables in a preliminary study. BJU Int 2008;101:1542–1546.
- Schultheiss D, Machtens S, Jonas U. Testosterone therapy in the ageing male: what about the prostate? Andrologia 2004;36:355–365.