Abstract
Objective: The bladder can be considered a target organ for testosterone action, and low testosterone levels possibly cause damage to bladder cells. We set out to study whether hypoandrogenism influences bladder wall cell damage in castrated and senile male rats. Methods: Thirty male Wistar rats were divided into three groups of 10 animals each: group I (3 months old), sham animals; group II (27 months old), senile animals; group III (3 months old), subjected to bilateral orchiectomy, and sacrificed 8 weeks after the procedure. Bladders were excised, weighed and sent for analysis. Stereological assays on collagen fibers and immunohistochemical analysis with active 3-caspase were performed on bladder cells. Results: Bladder weights were greater in the senile group than in the others. Stereological collagen fiber analysis demonstrated higher density in group III than in groups I and II (p < 0.05). The absolute density was 4.15 mm3 in group I, 22.3 mm3 in group II and 19.3 mm3 in group III. Semiquantitative active 3-caspase analysis showed greater percentages in the senile group II than in groups I and III. Conclusions: We can state that low plasma testosterone levels are related to higher collagen fiber density and active 3-caspase percentages in the bladder walls of orchiectomized and senile rats, respectively.
Keywords::
Introduction
Androgen receptors have been found in urethral and bladder epithelium [Citation1]. Studies have presented the role of testosterone on the autonomic reflex activity of the pelvic nervous system in rats [Citation2], and have suggested that testosterone influences nongenomic postsynaptic receptors that decrease or stop detrusor activity [Citation3,Citation4].
The detrusor presents age-related changes, and the composition and distribution of fibers in the bladder wall also become modified as the bladder ages [Citation5]. Lorenzetti et al. (2007) suggested that aging promotes morphological and functional changes in different cells through oxidative stress. Their study found greater DNA fragmentation in the detrusor cells of senile female rats, in which high levels of apoptosis would be expected [Citation6].
Gong et al. (2009) showed that active 3-caspase expression increased after testosterone deprivation, showing that testosterone plays a role in the apoptosis process of different tissues [Citation7]. Active 3-caspase is an important proteolytic enzyme, and its expression is increased in dead tissues due to oxidative stress action, because of induced nuclei damage [Citation8].
From a broader perspective, the present study aimed to evaluate the distribution of fibrosis and apoptosis processes in the bladder wall in male rats and to describe the evolution of bladder aging in relation to low testosterone levels.
Materials and methods
Animals and diet
Thirty male Wistar rats (housed at the University’s animal facility under a regimen of 12-h light and 12-h dark cycling, with access to food and water ad libitum) were used in these experiments.
Animal groups
The animals were divided into three groups of 10 animals each: group I (sham): animals were 3 months old, and underwent cystectomy; group II: animals were 27 months old, and underwent cystectomy; group III: animals were 3 months old, underwent bilateral orchiectomy, and then underwent cystectomy 8 weeks later.
The sample size in each group (n = 10), with 95% confidence interval, was obtained by means of the following statistical calculation: ![]() . The formula for calculating the sample size for a reliable estimate of the average size is given by:
. The formula for calculating the sample size for a reliable estimate of the average size is given by: ![]() . In this, n is the number of individuals in the sample, Zα/2 is the critical value that corresponds to the desired confidence interval, σ is the standard deviation of the population studied, and E is the sampling error (the difference between the measured and the true value). For a confidence level of 95% (α = 0.05), the critical value Zα/2 is 1.96. The above equation requires knowledge of the population standard deviation σ. Assuming σ = 3 and accepting an error E = 2, the sample size of 10 rats would suffice.
. In this, n is the number of individuals in the sample, Zα/2 is the critical value that corresponds to the desired confidence interval, σ is the standard deviation of the population studied, and E is the sampling error (the difference between the measured and the true value). For a confidence level of 95% (α = 0.05), the critical value Zα/2 is 1.96. The above equation requires knowledge of the population standard deviation σ. Assuming σ = 3 and accepting an error E = 2, the sample size of 10 rats would suffice.
After starting the experimental protocol, the animals were sacrificed and venous blood was collected from the dorsal vein of the tail, in order to assay the testosterone levels. The bladders were quickly removed, fixed in formalin and embedded in paraffin for use in histological and immunofluorescence studies.
Testosterone measurement
Total testosterone was assayed by means of competitive radioimmunoassaying (testosterone direct radioimmunoassay kit; Immunotech, Rio de Janeiro, Brazil, cat. no. 1119). The assay was calibrated for values over 20 ng/mL.
Morphometric evaluation
Picrosirius staining was used for histological analysis of fibrosis, and for the remaining histological features, slides were stained with hematoxylin and eosin.
All samples were evaluated by the same pathologist. Collagen fibers were quantified using the stereological method to determine the three-dimensional features of the anatomical structures based on two-dimensional sections. This theory is based on Delesse’s principle, which states that the relationship between the surface area of the organ and the section through the structure is the same as that between the volume of the structure and volume of the entire organ. The volumetric density of collagen fibers was quantified stereologically by superimposing the M-36 grade system on the morphological image of the slide. The volumetric density corresponded to the relative concentration of the structure or tissue of the study sample. The formula V = (Pp/Pt) × 100% was used to calculate the volumetric density (V) of the collagen fibers, in which Pp is the number of points in the structure (collagen fibers) and Pt, the number of points tested (36 in this case). The measurement was obtained using an optical microscope, and the fibers were counted at ×400 magnification in 10 random microscope fields in each group [Citation9].
Statistical analyses
Analysis of variance was used to evaluate the volumetric density of the collagen fibers around the implanted material. In both tests, p < 0.05 was considered to indicate significant differences.
Determination of apoptosis (active 3-caspase)
To prepare tissue sections for immunostaining, paraffin clearing from the slides was performed by placing them under a fume hood overnight, at 60 °C. Following this, the paraffin was cleared with xylene for 30 min and the slides were moved to a fresh dish of xylene for additional 30 min. The slides were rinsed twice for 10 min in 80% alcohol (18:1:1 100% ethanol:100% methanol:100% isopropanol). The slides were then rinsed five times with fresh deionized water.
The antigen retrieval method consisted of placing the slides face up in an incubation tray and covering each section with 1% sodium dodecyl sulfate (SDS) in phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4 and 1.47 mM KH2PO4). They were then incubated for 5 min at room temperature, followed by three 5-min washes with PBS. Blocking was performed by immersing the slides in blocking buffer 1 (3% Molico®, diluted 1:10 in PBS, i.e., 3 g of fat-free cow’s milk Molico®) to 100 ml of PBS. Following this, the slides were incubated overnight at 4 °C.
To prepare the incubation with primary antibodies (rabbit anti-caspase 3), the sections were covered with blocking buffer 2 (3% Molico®, diluted 1:10 in PBS, plus 200 µl of Tween). The tissue sections were covered with primary antibody (rabbit anti-caspase-3; C8487) diluted 1:500 in blocking buffer 2, and were incubated at 37 °C for 1 h. The excess liquid was blotted from the slides, and the slides were then rinsed three times in PBS for 5 min each wash.
To prepare the incubation with secondary antibodies (chicken anti-rabbit IgG), the tissue sections were covered with secondary antibody (chicken anti-rabbit IgG–F0382) diluted 1:40 in blocking buffer 2, and were incubated at 37 °C for 1 h. The excess liquid was blotted from the slides, and the slides were then rinsed three times in PBS for 5 min each wash.
To prepare the counterstaining and viewing (nuclear staining), the slides were immersed for 15 min in 100 µl of propidium iodide solution, diluted 1:10 in PBS. The slides were rinsed in PBS for 5 min. Cover slips were placed on the sections in order to view them under a microscope using fluorescence [Citation10].
The active 3-caspase assay was qualitatively classified according to its intensity, as mild (reaction in 0–25% of the area), mild to moderate (25–50%), moderate to severe (50–75%) and severe (≥75% of the area).
Results
At the time of sacrifice, the animals’ mean body weight was 268 g (252–310 g) in group I, 408 g (319–472 g) in group II and 272 g (248–300 g) in group III, and there was no statistical difference between them (p > 0.05). The bladders were then removed and weighed using Scherle’s method. The mean bladder weight was 62 g (58–67 g) in group I, 130 g (124–135 g) in group II and 61 g (55–64 g) in group III; there was no statistical difference between them (p > 0.05). The relationship between bladder weight and body weight was 0.23 in group I, 0.31 in group II and 0.22 in group III; p < 0.05 in group II compared with the others.
The serum testosterone concentrations immediately before sacrificing the animals were 53 ng/ml in group I, and <20 ng/ml in the orchiectomized and senile groups, i.e., below the lower limit of sensitivity of the method applied. This demonstrated the presence of a significant hormone deficit at the time of sacrificing these animals.
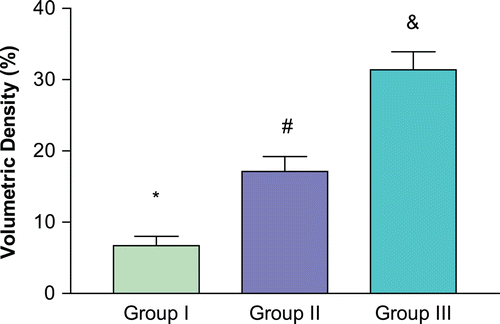
Volumetric density of collagen fibers
The graph in demonstrates the mean volumetric density of collagen fibers in the different groups. Greater fiber concentration was observed in the orchiectomized group than in the other groups.
Semiqualitative analysis of active 3-caspase in the bladder wall
The semiqualitative analysis of active 3-caspase has been shown in . The statistical analysis suggested that there was greater presence of 3-caspase in group II (senile) than in the other groups (p < 0.05). There was no percentage difference between groups I and III.
Table I. Semiqualitative analysis of active 3-caspase.
Discussion
The increasing morbidity of aging males involves many different systems, and frequently exerts direct and indirect actions on the lower urinary tract, promoting storage and obstruction symptoms. Roberts et al. (1998) demonstrated the presence of lower urinary tract symptoms ranging from 8–31% in men aged 50-years-old, increasing to 44% in men aged 70 or over [Citation11].
Several studies have correlated lower urinary tract symptoms and low testosterone levels in elderly men [Citation12,Citation13], but there have been few studies seeking to establish whether bladder dysfunction might be associated with sex hormones [Citation14]. Androgens, particularly testosterone and 5-α-dihydrotestosterone, have several functions during embryogenesis and in adulthood. However, these sex hormones present wide-ranging characteristics, possibly explained by differences in receptor-binding mechanisms.
Androgen signaling is amplified when testosterone converts to dihydrotestosterone in target cells. The testosterone-receptor nuclear complex differs from the dihydrotestosterone-receptor complex in that the latter presents longer lifetime and promotes androgen action that lasts longer, because of lower levels of oxidation-reduction reactions. Such reactions are responsible for androgen inhibition in target cells by dihydrotestosterone, but further studies are needed to elucidate how sex hormones influence the lower urinary tract [Citation15].
Testosterone effects are modulated in two ways. First, through nuclear receptors, which present activity levels that depend on DNA expression and the different receptor locations in organs. This is known as genomic hormone action. Second, through nonreceptor routes, which involve indirect mechanisms such as anti-inflammatory action, platelet antiaggregation and action on calcium and potassium channels.
Changes to sex hormone levels may promote an inflammatory process. The high prevalence of pelvic pain syndrome during aging is associated with decreasing testosterone levels, which could suggest that the anti-inflammatory action of testosterone is lost [Citation11]. In another possibility, Brann et al. (1989) [Citation16] demonstrated a relationship between low levels of prolactin and suppression of the anti-inflammatory action of testosterone and dihydrotestosterone without hypogonadism. They believed that the anti-inflammatory action of sex hormones could be modulated from low prolactin levels. On the other hand, inhibition of prolactin is not the only mechanism capable of decreasing the anti-inflammatory action of androgens.
Zhou et al. (1995) demonstrated that the action of dihydrotestosterone was stronger than that of testosterone [Citation17]. Studies in vivo have shown that the dissociation index of androgen receptors was three times faster with testosterone than with dihydrotestosterone, and the testosterone concentration needed to be 10 times higher than the dihydrotestosterone concentration for receptor stability to be maintained [Citation18].
In the present study, it was observed that rapidly decreasing testosterone levels were associated with high concentrations of collagen fibers in the bladder wall. It is clear that decreased androgen levels can inhibit the differentiation of smooth muscle [Citation19]. Androgen can stimulate stromal cells to differentiate in smooth muscle. Considering Traish et al. study [Citation19], and the association of results found in the present study, this may suggest that the decreasing testosterone levels were able to promote increases in the volumetric density of collagen fibers.
One explanation for increasing fibrotic process seen in the bladder of castrated rats would be the inability/incapacity of stromal cells to differentiate in muscle. This process could be partially due both to the genomic function (according to receptor) and to the anti-inflammatory nongenomic function of testosterone, thereby enabling higher concentrations of fibrosis in the bladder wall at times when there are rapid decreases in testosterone levels.
Celayir et al. demonstrated in a clinical study that the maximum bladder capacity and compliance decreased in patients with low testosterone levels. This evidence suggests that testosterone replacement or prophylaxis for hypogonadism could avoid or decrease the extent of lower urinary tract symptoms through maintenance of the complex collagen fiber/muscle fiber equilibrium [Citation20].
However, it must be noted that in the present study, there was a markedly greater fibrotic process with rapid testosterone decrease in the orchyectomized group than in the senile rats (slow decrease). This suggests that bladder dysfunction due to testosterone effects would occur in men as hypogonadism developed, with rapid suppression of testosterone.
In this study, it was observed that the senile group showed a greater percentage of apoptosis, as represented by the immunohistochemical marker 3-caspase, and lower volumetric density of collagen fibers than in the castrated group. Gong et al. (2009) [Citation7] observed increased expression of apoptosis markers in stained Leydig cells following testosterone deprivation, thus demonstrating the action of testosterone on cell apoptosis process in different tissues.
Corradi et al. (2004) [Citation21] showed that lower levels of dihydrotestosterone achieved through using finasteride promoted increases in collagen fiber concentration in prostate tissue. This may suggest that testosterone is strongly linked to stromal response, thereby changing the cell density of connective tissue, as also suggested by the present study.
It is possible that the greater percentage of apoptosis in the senile rats than in the castrated group was because the apoptosis signaling differed between the two groups. Schuster and Krieglstein suggested that TGF-β1 would promote apoptosis in senile animals, which could not be present in the sham group or in another situation in which there was a rapid decrease in testosterone [Citation22].
It is also possible that rat bladders are unable to express apoptosis levels sufficiently to be detected. In this way, higher levels of apoptosis in senile rats than in young rats would occur because the bladder tissue was incapable of expressing caspase adequately, compared with prostate tissue, for instance.
The difference between the groups can be explained in terms of time of exposure in the castrated group (8 weeks), or greater sensitivity of bladder tissue because of decreasing testosterone levels in the senile rats. Another possibility would be greater presence of free radicals in situations of slower testosterone decrease, such as in the case of senile rats.
Conclusion
The present study has shown that testosterone deprivation plays an important role in bladder structure, such as increasing of fibrosis levels and cell apoptosis in orchiectomized and senile rats, respectively.
Based on our results, we suggest that it would be very useful to develop a line of investigation in which the role of testosterone on bladder dysfunction is studied. Thus, the actual interactions between lower urinary tract symptoms, testosterone levels and aging among men could be evaluated.
Declaration of Interest: The authors declare no conflict of interest.
References
- Rosenzweig BA, Bolina PS, Birch L, Moran C, Marcovici I, Prins GS. Location and concentration of estrogen, progesterone, and androgen receptors in the bladder and urethra of the rabbit. Neurourol Urodyn 1995;14:87–96.
- Keast JR. The autonomic nerve supply of male sex organs–an important target of circulating androgens. Behav Brain Res 1999;105:81–92.
- Watkins TW, Keast JR. Androgen-sensitive preganglionic neurons innervate the male rat pelvic ganglion. Neuroscience 1999;93:1147–1157.
- Hall R, Andrews PL, Hoyle CH. Effects of testosterone on neuromuscular transmission in rat isolated urinary bladder. Eur J Pharmacol 2002;449:301–309.
- Masick JM, Levin RM, Hass MA. The effect of partial outlet obstruction on prostaglandin generation in the rabbit urinary bladder. Prostaglandins Other Lipid Mediat 2001;66:211–219.
- Lorenzetti F, Dambros M, Castro M, Ribeiro ML, Miranda DD, Ortiz V. Influence of oxidative stress and alpha tocopherol supplementation on urothelial cells of the urinary bladder in ovariectomised rats. Int Urogynecol J Pelvic Floor Dysfunct 2007;18:1351–1356.
- Gong YG, Wang YQ, Gu M, Feng MM, Zhang W, Ge RS. Deprival of testicular innervation induces apoptosis of Leydig cells via caspase-8-dependent signaling: a novel survival pathway revealed. Biochem Biophys Res Commun 2009;382:165–170.
- Leewenburgh C, Pollack M, Phaneu JS The role of apoptosis int the normal aging brain, skeletal muscle, and heart. NY Acad Sci 2002;959:93–107.
- Thiel M, Rodrigues Palma PC, Riccetto CL, Dambros M, Netto NR Jr. A stereological analysis of fibrosis and inflammatory reaction induced by four different synthetic slings. BJU Int 2005;95:833–837.
- Ludwig L, Seraphim D, Lorenzetti F, Bertolla RP, Ortiz V, Dambros M. Inhibitory activity of a-tocopherol on apoptosis in the rat bladder wall subjected to androgen deprivation. Neurourol Urodyn 2011;30:194–198.
- Roberts RO, Lieber MM, Rhodes T, Girman CJ, Bostwick DG, Jacobsen SJ. Prevalence of a physician-assigned diagnosis of prostatitis: the Olmsted County Study of Urinary Symptoms and Health Status Among Men. Urology 1998;51:578–584.
- Yassin AA, El-Sakka AI, Saad F, Gooren LJ. Lower urinary-tract symptoms and testosterone in elderly men. World J Urol 2008;26:359–364.
- Haidinger G, Temml C, Schatzl G, Brössner C, Roehlich M, Schmidbauer CP, Madersbacher S. Risk factors for lower urinary tract symptoms in elderly men. For the Prostate Study Group of the Austrian Society of Urology. Eur Urol 2000;37:413–420.
- Liu YH, Qi J, Hou YX, Wang F. Effects of sex hormones on genioglossal muscle contractility and SR Ca2+-ATPase activity in aged rat. Arch Oral Biol 2008;53:353–360.
- Lindzey J, Kumar MV, Grossman M, Young C, Tindall DJ. Molecular mechanisms of androgen action. Vitam Horm 1994;49:383–432.
- Brann DW, Rao IM, Mahesh VB. Antagonism of estrogen-induced prolactin release by progesterone. Biol Reprod 1988;39:1067–1073.
- Zhou ZX, Lane MV, Kemppainen JA, French FS, Wilson EM. Specificity of ligand-dependent androgen receptor stabilization: receptor domain interactions influence ligand dissociation and receptor stability. Mol Endocrinol 1995;9:208–218.
- Wright AS, Thomas LN, Douglas RC, Lazier CB, Rittmaster RS. Relative potency of testosterone and dihydrotestosterone in preventing atrophy and apoptosis in the prostate of the castrated rat. J Clin Invest 1996;98:2558–2563.
- Traish AM, Goldstein I, Kim NN. Testosterone and erectile function: from basic research to a new clinical paradigm for managing men with androgen insufficiency and erectile dysfunction. Eur Urol 2007;52:54–70.
- Celayir S. Effects of different sex hormones on male rabbit urodynamics: an experimental study. Horm Res 2003;60:215–220.
- Corradi LS, Góes RM, Carvalho HF, Taboga SR. Inhibition of 5-alpha-reductase activity induces stromal remodeling and smooth muscle de-differentiation in adult gerbil ventral prostate. Differentiation 2004;72:198–208.
- Schuster N, Krieglstein K. Mechanisms of TGF-beta-mediated apoptosis. Cell Tissue Res 2002;307:1–14.