Abstract
Objectives: Tamsulosin is an alpha-1 adrenoceptor antagonist applied in treating lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH). This study aimed to evaluate safety, efficacy and acceptance of newly formulated orally disintegrating tamsulosin tablets in Taiwanese patients with LUTS/BPH. Methods: This single center, non-comparative, observational study enrolled 45 male patients over age 50 years. All patients received 0.2 mg tamsulosin orally disintegrating tablets daily and were evaluated at weeks 0, 4, 8, 12 of the 12-week treatment period. Tamsulosin efficacy was evaluated by International Prostate Symptom Score (I-PSS) with 7 questions on urinary symptoms and one disease-specific quality-of-life question, with scores ranging from 0 (no symptoms) to 35 (highly symptomatic). Maximum flow rate (ml/s), voided volume (ml), flow time (s), and mean flow rate (ml/s) were measured. Danish prostatic symptom sexual function scale rated severity and associated concerns of erection quality, ejaculatory function and pain/discomfort were also assessed. Results: Patients’ mean ± SD age was 62.47 ± 7.77 (range: 50–89) and mean ± SD I-PSS was 13.98 ± 5.50. Statistically significant changes from baseline were found in post-test I-PSS and quality of life (both P < 0.001). Mean ± SD I-PSS decreased from 14.30 ± 9.34 to 6.73 ± 0.88 at patients’ final visit. Statistically significant increases in mean maximum flow rate and mean flow rate were found over 12-week study period (P < 0.05). No adverse events were reported. No significant differences were found in pulse, SBP/DBP or sexual function. Conclusion: Orally disintegrating tamsulosin tablets demonstrate acceptable safety and efficacy for acceptance and well tolerance by Taiwanese LUTS/BPH patients.
Introduction
Benign prostatic hyperplasia (BPH) is a common condition among older adult males, occurring in up to 70% of men over age 60 years [Citation1]. Prevalence of BPH in Taiwanese men over age 50 years is reported to be as high as that of Western countries [Citation2]. BPH commonly results in bladder outlet obstruction and lower urinary tract symptoms (LUTS). About half of men with BPH develop LUTS and incidence increases linearly with age until age 79 years [Citation1]. LUTS/BPH may be the result of benign prostatic obstruction (BPO) caused by an enlarged prostate (static or mechanic component) induced by an increase in the number of prostatic cells [Citation3]. It may also be due to obstruction caused by an increased contraction of smooth muscle cells in the prostate, urethra and bladder neck due to stimulation of α-1-adrenoceptors (dynamic component) [Citation4]. Besides obstruction, increasing evidence indicates that other factors such as detrusor hypocontractility, central nervous system disorders, aging and/or ischemia may contribute to the development of LUTS/BPH [Citation3]. The long-term consequences may include acute and chronic urinary retention, recurrent urinary tract infections, gross hematuria and bladder stones, any of which may require hospital admission and/or surgery [Citation5].
Typically, patients with LUTS/BPH first seek medical advice because they are bothered by their symptoms. Although voiding symptoms such as weak stream, hesitancy, intermittency, dribbling, abdominal straining and incomplete bladder emptying are prevalent, patients report that storage symptoms such as frequency, nocturia, urgency and urge incontinence are the most bothersome [Citation6]. Storage symptoms interfere to the greatest extent with daily life activities and have a major impact on patients’ quality of life; and interference with sexual function and erection is reported by patients to be more bothersome than ejaculation problems [Citation7].
Treatment for LUTS/BPH includes surgery or medical therapy such as α-1-adrenoceptor antagonists and 5 α-reductase inhibitors. Medical therapy is the mainstay of care for most men with LUTS since surgery is invasive, time consuming and associated with considerable irreversible morbidity such as impotence and incontinence [Citation7]. In Italy, about 53% of men receive medical therapy while 37% receive surgery; α-1 adrenoceptor antagonists are the most frequently prescribed medical therapy (70%) [Citation7]. Treatment with α-1-adrenoceptor antagonists such as alfuzosin, doxazosin, and tamsulosin is recommended as first-line pharmacologic therapy for men with LUTS/BPH [Citation8]. Tamsulosin relieves obstruction and related voiding problems by relaxing smooth muscle in the prostate, urethra and bladder neck due to the blockade of α-1A-adrenoceptors in these tissues and subsequent increased concentration of α1-acid glycoprotein [Citation9]. Blockade of α-1A- and α-1D-adrenoceptors in the bladder and/or its innervating structures may be associated with relief of storage symptoms. Tamsulosin hydrochloride (brand name Flomax®) was developed by Astellas Pharma Inc., Tokyo, Japan, and has been approved and used in the United States, European countries, Japan and Taiwan for the treatment of BPH for many years. Of the currently available α-1-adrenoceptor antagonists, tamsulosin capsules are reported to have the most favorable tolerability/efficacy ratio [Citation10]. This is probably due to tamsulosin’s beneficial effects in relieving LUTS with minimal undesired side effects on the cardiovascular system.
A newly developed orally disintegrating tablet formulation of tamsulosin was approved for use in Taiwan in 2006. Orally disintegrating tablets (ODTs), often referred to as “fast melts,” disintegrate in the mouth in seconds without the need of chewing [Citation11]. This new dosage form can be taken without water, which makes it convenient and easy for patients to use, especially for older males or patients on restricted water intake. The safety and efficacy of the orally disintegrating formulation of tamsulosin has not yet been thoroughly evaluated in Taiwan.
This study aimed to evaluate the clinical efficacy, safety/tolerability and patients’ acceptance of the new tamsulosin formulation in Taiwanese patients with LUTS suggestive of BPH.
Materials and methods
Patients
This study was conducted at the Wan Fang Medical Center at Taipei Medical University from May 1, 2008 to March 31, 2009. A total of 45 male patients who regularly visited the outpatient urology clinic were enrolled. Patients were eligible for the study if they were a male patient aged ≧ 50 years, with clinical signs and symptoms of frequency and urgency related to BPH, an IPSS total score of ≧ 8 at baseline, maximum flow rate (Qmax) of 4–15 mL/sec, and currently not using orally disintegrating tablet of tamsulosin for the treatment of LUTS/BPH. Patients were excluded from participation if they had previous or planned prostate surgery, including transurethral resection of the prostate (TURP), transurethral microwave treatment (TUM), transurethral needle ablation (TUNA), laser, or other invasive or minimally invasive procedures within 12 months; had a neurological cause for abnormal detrusor activity, urinary tract infection, chronic inflammation, bladder stones, bladder neck sclerosis, urethral stricture, prostatic cancer, severe vesical diverticulum, severe hepatic dysfunction, severe renal dysfunction, severe cardiovascular disorder, or other disorders that investigators believed made the patients unsuitable for the trial; had received α-blockers, α/β-blockers, anti-androgens, 5 α-reductase inhibitors, cholinergic agents or anti-cholinergics, or had hypersensitivity to tamsulosin or to any component of the formulation, or taken any investigative drug in the 3 months prior to this study. The study protocol was reviewed and approved by the institutional review board of Taipei Medical University-Wan Fang Medical Center. All patients provided signed informed consent.
Study design and procedure
This was a single center, non-comparative, observational study. In the absence of a control group, it was considered to be a sufficiency trial designed to demonstrate that the tamsulosin activity was sufficient to meet the purpose of evaluating safety and efficacy of the drug. The treatment period was 12 weeks. Patients who met the inclusion criteria were given tamsulosin orally disintegrating tablet 0.2 mg daily and were evaluated on scheduled patient visits at weeks 0, 4, 8 and 12 of the treatment period. The initial dose of the orally disintegrating tablet of tamsulosin was 0.2 mg. However, the daily dose of the orally disintegrating tablet of tamsulosin could be adjusted up to 0.4 mg at follow-up visits according to the patient’s condition and investigator’s judgment. Data from the International Prostate Symptom Score (I-PSS) [Citation12] was used to evaluate the efficacy of tamsulosin. The Danish Prostatic Symptom Score (DAN-PSS1) [Citation13] was applied to help evaluate patients’ sexual function during treatment with tamsulosin as a possible indication of efficacy. Symptoms and quality of life (QOL) were monitored by patients’ responses to the I-PSS and DAN-PSS-1 questionnaires at each visit. Urinary parameters measured at each patient visit included maximum flow rate (ml/s), voided volume (ml) and flow time (s) measured by uroflowmetry; mean flow rate (ml/s) was calculated by dividing the voided volume (ml) by the flow time (s).
Instruments
International prostate symptom score (I-PSS)
The I-PSS is a screening tool used to screen, diagnose, monitor and help manage LUTS/BPH. It consists of 8 questions, including 7 questions concerning urinary symptoms and one on disease-specific quality-of-life [Citation12]. Symptoms questions include feeling of incomplete bladder emptying, frequency, intermittency, urgency, weak stream, straining and nocturia during the last month. A score between 0 and 5 is assigned to each response. The quality-of-life question is scored from 1 to 6. Possible total scores range from 0 (no symptoms) to 35 (highly symptomatic).
Danish prostatic symptom score (DAN-PSS-1)
The DAN-PSS-1 [Citation13] is a self-administered, disease-specific quality-of-life scale administered to evaluate LUTS suggestive of BPH. It consists of 12 questions about symptoms of bladder storage and voiding and the degree to which each symptom affects the daily life of (bothers) individual patients. Questions include the associated bother of three sexual symptoms: quality of erection, ejaculatory function, and pain/discomfort on ejaculation. The scale measures symptom scores and bother scores as a “bother index,” that is, a ratio of the number of men with higher bother scores than symptoms scores compared to those with higher symptom scores than bother scores. The scale has been recognized as reliable, valid and responsive and has been widely used to assess symptoms and monitor treatment in patients with LUTS/BPH [Citation14].
Sample size calculation
Prior data indicate that the difference in the I-PSS total score of matched pairs is normally distributed with standard deviation of 5.06 [Citation15]. If the true difference in the I-PSS total score is 7.5, 33 matched pairs of subjects would need to be studied to reach the conclusion of the rejection to null hypothesis that this response difference is zero with the power of 99.9%. A sample size of 45 patients is necessary given an anticipated dropout rate of 36%. The Type I error probability associated with this test of null hypothesis is 0.05.
Statistical analysis
Demographic and clinical characteristics at baseline were analyzed for all patients enrolled. Demographics and baseline characteristics are represented as means ± standard deviations (SD). Primary endpoints (I-PSS and quality of life) and secondary endpoints (uroflowmetry) were analyzed based on the intent-to-treat (ITT) population, which was defined as all patients who received at least one dose of the study medication and had at least one post-baseline on-treatment visit. Repeated measurements ANOVA was carried out to assess the time trend during the study period and data are shown as means ± standard errors (SE). An endpoint visit was analyzed to account for patients prematurely terminating the study, lost observation carried forward (LOCF) technique was applied for missing values. For all analyses, a two-sided P value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS 15.0 statistics software (SPSS Inc, Chicago, IL).
Results
A total of 45 patients with BPH were enrolled in the study. The ITT population was 33 because 12 patients (26.7%) of 45 screened were unable to complete baseline measurements. Finally, after 5 more patients dropped out during the study, only 28 patients (62.2%) completed the study. Patients’ mean ± SD age was 62.47 ± 7.77 (range: 50–89) and the mean ± SD I-PSS was 13.98 ± 5.50. Patients’ demographics and baseline characteristics are shown in .
Table I. Patients’ demographics and baseline characteristics (n = 45).
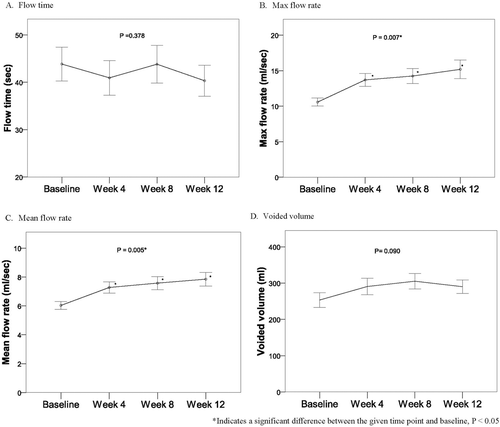
describes the changes from baseline in primary endpoints (I-PSS and quality of life) at the end of the 12-week trial with tamsulosin orally disintegrating tablet in the ITT population. Over the study period, statistically significant changes from baseline were found in I-PSS and quality of life (both P < 0.001). Total symptom score of I-PSS was reduced from 14.30 ± 9.34 to 6.73 ± 0.88 at the final visit of patients receiving orally disintegrating tamsulosin tablets. The secondary endpoints for changes from baseline in uroflowmetry during the study period are shown in . Statistically significant increases from baseline in maximum flow rate and mean flow rate were found over the study period (P < 0.05). However, no significant differences were found in flow time and voided volume during the whole 12-week treatment period.
Figure 1. Primary endpoints (I-PSS and Quality of life) in LUTS/BPH ITT population during 12-weeks administration of tamsulosin. *Significant difference between the given time point and baseline, P < 0.05.
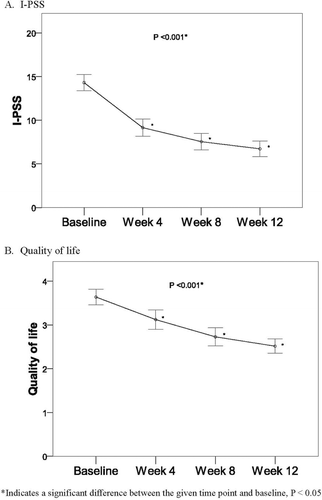
Figure 2. Secondary endpoints (uroflowmetry) in LUTS/BPH patients during 12-week tamsulosin administration. *Significant difference between the given time point and baseline, P < 0.05.
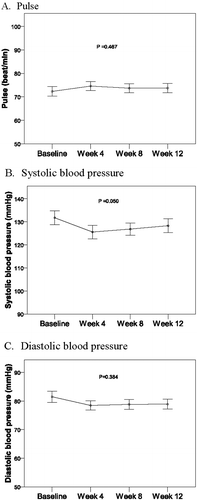
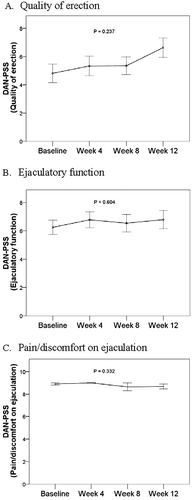
Assessments of drug safety included adverse events, vital signs and sexual function. No adverse events were reported in any patient. No statistically significant differences in pulse, SBP, DBP and were found during the study period (). The DAN-PSS was used to evaluate the severity and the associated bother of three sexual symptoms: quality of erection, ejaculatory function, and pain/discomfort on ejaculation. There were no significant differences in patients’ sexual function from baseline measurement to the end of the study period ().
Discussion
After 33 male patients with urinary tract symptoms suggestive of BPH received treatment with orally disintegrating tamsulosin tablets for 12 weeks, statistically significant differences were found in I-PSS and QOL compared to baseline scores. The mean ± SD total symptom score of I-PSS was reduced from 14.30 ± 9.34 to 6.73 ± 0.88 at patients’ final visit. The secondary endpoints for changes from baseline in uroflowmetry during the study period demonstrated statistically significant increases in maximum flow rate and mean flow rate, but no significant changes from baseline were found in flow time and voided volume during the whole 12-week treatment period. Results of the DAN-PSS, which was used to evaluate the severity and the associated bother of quality of erection, ejaculatory function, and pain/discomfort on ejaculation, revealed no significant changes from baseline in patients’ sexual function during the study period. No adverse events were reported in any patient during treatment and no statistically significant changes from baseline in pulse, SBP, DBP and were found between baseline and the 12-week endpoint.
Our results for the newly formulated orally disintegrating tamsulosin tablet compare favorably to those of other studies demonstrating the safety and efficacy of tamsulosin. Although the safety and efficacy of tamsulosin is well established, the recent release of generic tamsulosin hydrochloride drugs prompted further study. A multicenter controlled trial compared the safety and efficacy of two generic α-1-adrenoceptor antagonists (Sulosin D and Harnal D) in equal groups of LUTS/BPH patients, demonstrating non-inferiority of Sulosin D to Harnal D [Citation16]. Mean changes in I-PSS, QOL index and post-voiding residuals (PVR) were comparable between the two groups; and significant decreases were noted in I-PSS from baseline to endpoint as they were in our study of tamsulosin. In the multicenter study, the within-group changes were statistically significant in both groups, indicating perceptible improvement in patients’ symptoms and efficacy of the two generic drugs. In our study, the changes in the IPSS from baseline to endpoint represent the primary measure of the orally disintegrating tamsulosin formulation efficacy. The QOL subscale of the IPSS was also improved in the present study, corresponding to symptom improvement in both voiding and storage parameters.
A long-term study (24 months) evaluating the effects of tamsulosin treatment on changes in frequency-volume chart (FVC) data, especially nocturia, in BPH patients found that tamsulosin reduced nighttime urine production over the long-term study period [Citation17]. Similar to our study, all patients completed the IPSS, QOL index and uroflowmetry at enrollment and at each scheduled visit for the first 12 weeks of the study. IPSS, QOL and maximum flow rate improved over the initial 12-week period as they did in the present 12-week study. In our study, the individual nocturia scores of the IPSS were also among the improved results. Although the present study evaluated voided volume and found no differences from baseline to the final measurement at 12 weeks, this result alone is not sufficient to indicate definitive changes in daytime or nighttime urine production. Because nocturia is common in BPH and may affect quality of life, further study is needed to evaluate the effects of tamsulosin orally disintegrating tablets on nocturia. Twenty-four hour FVC was not used in the present study but is an effective tool for assessing nocturia and should be employed in future study.
Combination drug therapy is another approach that has been investigated for the treatment of LUTS/BPH [Citation18–20]. A review of combination therapies for managing LUTS/BPH indicated that combinations of α1-adrenergic antagonist and 5 α-reductase inhibitors are supported for the treatment of symptomatic BPH and that studies consistently found superior results with combined therapies than with tamsulosin alone [Citation18]. Bechara et al. [Citation19] demonstrated improved IPSS and QOL subscale with tamsulosin and tamsulosin plus tadalafil, but greater improvement was seen in patients receiving the combination of drugs than in those receiving tamsulosin alone. A recent trial compared the safety and efficacy of tamsulosin alone with tamsulosin and vardenafil, another established drug for treating LUTS/BPH. The combination proved to be well tolerated and more effective in improving LUTS and erectile function than tamsulosin alone [Citation20]. In that study, results of the International Index of Erectile Dysfunction (IEFF) revealed that improvements in erectile function with combination drugs were more remarkable than with tamsulosin alone. In the present study, using the three sexual function parameters of the DAN-PSS-1 (quality of erection, ejaculatory function, and pain/discomfort on ejaculation) to evaluate sexual function in patients receiving tamsulosin orally disintegrating tablets, there were no significant differences in patients’ sexual function from baseline to endpoint. This may be explained by either a lack of acuity of these symptoms in the study participants, incomplete results of the instrument (i.e. use of only three parameters) or the short term of the study period. Further study of the orally disintegrating formulation of tamsulosin is necessary and comparison with other established drug therapies for LUTS/BPH would likely be of value.
Besides sexual function results, the safety of orally disintegrating tamsulosin tablets was demonstrated in the present study through monitoring of adverse events and measurement of vital signs. Notably, no adverse events were reported in any patient and no statistically significant differences in pulse, SBP and DBP and were found during the study period. It has been demonstrated that the activity of α-1-adrenergic antagonists directed to the lower urinary tract also affects the vasculature and may cause cardiovascular side effects [Citation21]. The lack of cardiovascular side effects is one reason for the overwhelming support of tamsulosin as medical treatment for LUTS/BPH. This study did not evaluate cardiac function at baseline or in the scheduled visits during the trial, and patients with advanced cardiovascular disease were excluded, but no changes in blood pressure were noted that would indicate adverse cardiovascular effects, which is an important indicator of tolerability.
The abnormal ejaculation of semen is a typical but infrequent side effect of some α-1-adrenoceptor antagonists such as silodosin or tamsulosin [Citation22]. Recent clinical studies suggested that this represents a relative anejaculation rather than a retrograde ejaculation for tamsulosin (0.8 mg) [Citation23]. In contrast, anejaculation was not observed in any subjects in the alfuzosin or placebo groups [Citation24]. Goktas et al. (2006) reported an interesting study that intermittent tamsulosin treatment on abnormal ejaculation was well tolerated and provided comparable improvements for abnormal ejaculation while compared patients received a 0.4 mg tamsulosin capsule daily with patients with abnormal ejaculation received 0.4 mg tamsulosin once daily every other day [Citation25]. In our study, patients were given tamsulosin orally disintegrating tablet 0.2 mg daily and had no retrograde ejaculation side effects. It may be attributed to low dose of tamsulosin we used.
In any formulation, the pharmacokinetics of tamsulosin are not affected by age and mild-to-moderate alterations in the pharmacokinetics in patients with renal or hepatic impairment are related to tamsulosin’s high plasma-protein binding to α1-acid glycoprotein [Citation9]. Although the pharmacokinetics and pharmacodynamics of modified-release tamsulosin and oral controlled absorption system formulations have demonstrated treatment efficacy with fewer cardiovascular effects, the orally disintegrating formulation may need additional study to demonstrate equivalent performance. Meanwhile, in our study, patients readily accepted the orally disintegrating tablet and expressed satisfaction with the results of their treatment.
A limitation of this study is that no control group or placebo group was used for comparison, which would have provided additional comparative data to support safety and efficacy. Also, the 12-week term of the study was sufficient to demonstrate efficacy and safety/tolerability but was not sufficient to demonstrate significant long-term changes in voiding parameters such as voided volume. A longer term study with a larger sample and a control group is warranted to confirm the results of the present study and demonstrate long-term efficacy and safety of tamsulosin orally disintegrating tablet formulation.
Conclusion
Orally disintegrating tamsulosin tablets demonstrate acceptable safety and efficacy and are readily accepted and well tolerated by Taiwanese LUTS/BPH patients.
Declaration of Interest: This work was supported by DOH100-TD-B-111-003. The authors report no conflicts of interest.
References
- Verhamme KM, Dieleman JP, Bleumink GS, van der Lei J, Sturkenboom MC, Artibani W, Begaud B, et al.; Triumph Pan European Expert Panel. Incidence and prevalence of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in primary care–the Triumph project. Eur Urol 2002;42:323–328.
- Lai MK. Recent advances in the treatment of benign prostatic hyperplasia. J Formos Med Assoc 1996;95:822–827.
- Artibani W. Introduction. Eur Urol 2001;40 Suppl 4:1–4.
- Michel MC, Vrydag W. Alpha1-, alpha2- and beta-adrenoceptors in the urinary bladder, urethra and prostate. Br J Pharmacol 2006;147 Suppl 2:S88–119.
- Emberton M, Cornel EB, Bassi PF, Fourcade RO, Gómez JM, Castro R. Benign prostatic hyperplasia as a progressive disease: a guide to the risk factors and options for medical management. Int J Clin Pract 2008;62:1076–1086.
- Peters TJ, Donovan JL, Kay HE, Abrams P, de la Rosette JJ, Porru D, Thüroff JW. The International Continence Society “Benign Prostatic Hyperplasia” Study: the botherosomeness of urinary symptoms. J Urol 1997;157:885–889.
- Scarpa RM. Lower urinary tract symptoms: what are the implications for the patients? Eur Urol 2001;40 Suppl 4:12–20.
- AUA Practice Guidelines Committee. AUA guideline on management of benign prostatic hyperplasia. J Urol 2003;170:530.
- Franco-Salina G, de la Rosette JJ, Michel MC. Pharmacokinetics and pharmacodynamics of tamsulosin in its modified-release and controlled absorption system formulations. Clinical Pharmacokinetics 2010; 49:177.
- Djavan B, Chapple C, Milani S, Marberger M. State of the art on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Urology 2004;64:1081–1088.
- Badgujar BP, Mundada AS. The technologies used for developing orally disintegrating tablets: a review. Acta Pharm 2011;61:117–139.
- International Prostate Symptom Score (I-PSS). Urological Sciences Research Foundation. http://www.usrf.org/questionnaires/AUA_SymptomScore.html.
- Meyhoff HH, Hald T, Nordling J, Andersen JT, Bilde T, Walter S. A new patient weighted symptom score system (DAN-PSS-1). Clinical assessment of indications and outcomes of transurethral prostatectomy for uncomplicated benign prostatic hyperplasia. Scand J Urol Nephrol 1993;27:493–499.
- Hansen BJ, Flyger H, Brasso K, Schou J, Nordling J, Thorup Andersen J, Mortensen S, et al. Validation of the self-administered Danish Prostatic Symptom Score (DAN-PSS-1) system for use in benign prostatic hyperplasia. Br J Urol 1995;76:451–458.
- Li NC, Chen S, Yang XH, Du LD, Wang JY, Na YQ; and The Beijing Tamsulosin Study Group. Efficacy of low-dose tamsulosin in chinese patients with symptomatic benign prostatic hyperplasia. Clin Drug Investig 2003;23:781–787.
- Lee HS, Kim SW, Oh SJ, Choo MS, Lee KS. Efficacy and safety of tamsulosin for treating lower urinary tract symptoms associated with benign prostatic hyperplasia: a multicenter, randomized, controlled, open-label non-inferiority study. Korean J Urol 2012;53:178–183.
- Kojima Y, Sasaki S, Imura M, Kubota Y, Hayashi Y, Kohri K. Tamsulosin reduces nighttime urine production in benign prostatic hyperplasia patients with nocturnal polyuria: a prospective open-label long-term study using frequency-volume chart. Neurourol Urodyn 2012;31:80–85.
- Cohen SA, Parsons JK. Combination pharmacological therapies for the management of benign prostatic hyperplasia. Drugs Aging 2012;29:275–284.
- Bechara A, Romano S, Casabé A, Haime S, Dedola P, Hernández C, Rey H. Comparative efficacy assessment of tamsulosin vs. tamsulosin plus tadalafil in the treatment of LUTS/BPH. Pilot study. J Sex Med 2008;5:2170–2178.
- Gacci M, Vittori G, Tosi N, Siena G, Rossetti MA, Lapini A, Vignozzi L, et al. A randomized, placebo-controlled study to assess safety and efficacy of vardenafil 10 mg and tamsulosin 0.4 mg vs. tamsulosin 0.4 mg alone in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Sex Med 2012;9:1624–1633.
- Barendrecht MM, Koopmans RP, de la Rosette JJ, Michel MC. Treatment of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: the cardiovascular system. BJU Int 2005; 95(Suppl 4):S19–S28.
- Michel MC. Alpha1-adrenoceptors and ejaculatory function. Br J Pharmacol 2007;152:289–290.
- Hisasue S, Furuya R, Itoh N, Kobayashi K, Furuya S, Tsukamoto T. Ejaculatory disorder caused by alpha-1 adrenoceptor antagonists is not retrograde ejaculation but a loss of seminal emission. Int J Urol 2006;13:1311–1316.
- Hellstrom WJ, Sikka SC. Effects of acute treatment with tamsulosin versus alfuzosin on ejaculatory function in normal volunteers. J Urol 2006;176:1529–1533.
- Goktas S, Kibar Y, Kilic S, Topac H, Coban H, Seckin B. Recovery of abnormal ejaculation by intermittent tamsulosin treatment. J Urol 2006;175:650–2; discussion 652.