Abstract
Background: Symptoms of the “male climacteric” are often at least in part referred to an age-dependent decline of serum androgen levels. Therefore, we evaluated the relationship of climacteric symptoms as assessed by the “Aging Males’ Symptoms” (AMS) Questionnaire with circulating androgen levels. Methods: 146 ambulatory men (age, 27–85 years) were surveyed with the AMS Questionnaire and sampled for serum values of total testosterone (tT) and sexual hormone binding globulin (SHBG). Free testosterone (fT) was calculated from tT and SHBG. A total AMS score ≥37 was considered pathological; the lower limits for tT and fT were set to 8 nmol/l and 180 pmol/l, respectively. Results: A significant deficit in tT and fT was shown in 25 (17.1%) and 34 (24.5%) men, respectively; the AMS Questionnaire showed pathological results for 66 (45.2%) men. In predicting a tT deficit, the AMS Questionnaire rendered a sensitivity of 76% and a specificity of 61.6%, only. However, multiple regression analysis revealed a significant correlation of lowered tT with a pathological somatovegetative and psychological AMS subscore (p = 0.042 and p = 0.01) and a correlation of lowered fT with a pathological sexual subscore (p = 0.039). Conclusion: In predicting hypogonadism the AMS Questionnaire in total did not render a sufficient diagnostic efficiency.
Introduction
The circulating serum androgen levels in men often decrease with age. In men aged ≥60 years longitudinal studies have shown a decline of total testosterone (tT) by about 2% per year [Citation1–3]. Due to an increase in sexual hormone binding globulin (SHBG) which appears with aging, the decline of the unbound free testosterone (fT) can be even more pronounced [Citation1,Citation4,Citation5]. The mechanisms of gradual androgen depletion in the aging male are widely unknown. Most likely, the genesis is multifactorial, consisting of a reduced hypothalamic gonadotropin releasing hormone outflow, decreased responsiveness of testicular tissue and alterations in androgenic negative feedback [Citation6]. For the diagnosis of hypogonadism in contrast to tT the role of fT is not fully understood, but existing guidelines recommend fT determination in addition tT if the latter is approximating the lower border of the reference range [Citation7,Citation8].
The age-related decrease of circulating androgens is paralleled by emerging climacteric symptoms in the elderly men [Citation9]. Typical symptoms are a decline in libido, reduction of physical strength, depressive mood and impaired cognitive functions. This assumption is supported by a number of clinical investigations. Accordingly, the replacement of testosterone in androgen-deficient men with sexual complaints can have a pronounced positive influence on sexual desire and erectile function [Citation10]. Furthermore, endogenous testosterone is responsible for maintaining lean body mass, physical strength, cognitive performance and euthymia [Citation11–14].
To facilitate the assessment of age-related symptoms, a questionnaire, “Aging Males’ Symptoms” (AMS) Scale was developed in the late 1990s by Heinemann et al. [Citation15]. The total AMS score is well acknowledged and was shown to have a good correlation with the severity of climacteric symptoms [Citation16,Citation17]. The questionnaire is today in widespread use and has up to now been translated into several languages [Citation18–22]. Originally developed for the assessment of age-related symptoms, over the last years the AMS Questionnaire has also been studied for its ability to predict a prevailing androgen deficit in men. A number of studies failed to reveal a correlation of the questionnaire results with serum testosterone levels [Citation23–25]. On the other hand, Morley et al. [Citation26] found a significant correlation of AMS scores with lowered fT and bioavailable testosterone. Similar results were published by Clapauch et al. [Citation27] for tT.
These controversial results encouraged us to evaluate AMS Questionnaire results of urological outpatients and correlate their results with circulating androgen levels. Moreover, we aimed to determine the AMS Questionnaire’s potential as a prediction tool for serologic hypogonadism in clinical practice.
Methods
Participants
Men included in this study were derived from a random sample of urologic outpatients of the University Hospital Ulm, Dept. of Urology. A total of 146 ambulatory men aged 27–85 were evaluated in this study. They were surveyed with the AMS Questionnaire, sampled for tT (146/146 men) and SHBG (139/146 men). Body mass index (BMI) was determined in 90 of 146 men. Informed consent was given by each patient for the scientific use of the collected data. The whole study was conducted in accordance with the principles of the Declaration of Helsinki.
AMS Questionnaire
The AMS Questionnaire was originally developed by Heinemann et al. [Citation15] to enable a convenient and reliable assessment of symptoms in the aging male. It consists of 17 questions and includes three dimensions of symptoms: (i) somatovegetative (7 items), (ii) psychological (5 items), and (iii) sexual symptoms (5 items). Each question is represented by a five-point Likert item (from 1 = “no symptoms” to 5 = “very severe symptoms”). Therefore, possible total scores range from 17 to 85 (17–26 points: no symptoms; 27–36 points: mild symptoms; 37–49 points: moderate symptoms; >50 points: severe symptoms). In our study, the total AMS score was considered significantly pathologic, if symptoms were moderate or severe (≥37 points). Moreover, AMS subscores of ≥15 points (somatovegetative) or ≥11 points (psychological, sexual) were considered pathologic.
Laboratory assays
Blood samples were collected from an anticubital vein of each subject between 8:00 and 11:00 a.m. and measured for tT and SHBG. Determination of tT and SHBG was performed by Electrochemiluminescence Immunoassay (ECLIA, ROCHE, Switzerland) in a MODULAR E170® equipment (ROCHE, Switzerland). FT was calculated from tT and SHBG by using the Vermeulen’s algorithm [Citation28]. Limits for tT and fT were set to ≥8 nmol/l and ≥180 pmol/l.
Statistics
Descriptive statistics are given either in medians or means + standard deviations. Statistical analysis was performed using commercial available software (SPSS Statistics, V.19.0). Spearman correlation coefficients were calculated for studying univariate associations for ordinal data and not normally distributed data. In order to evaluate the independent contribution of questionnaire results in explaining the variability of androgen levels multiple regression analyses were performed with correction for age and BMI. p values <0.05 were considered significant.
Results
AMS score results
The mean total AMS score in the studied population was 36.9 ± 12.7. Categorized into four groups, about every fourth patient had no climacteric symptoms according to the AMS Questionnaire, mild and moderate symptoms were both seen in 29.5% of the cases, 15.8% had severe symptoms according to the AMS Questionnaire (). Thus, 45.2% of surveyed men had a total AMS score of ≥37, which was considered as significantly pathologic in this study. The highest rate of pathological AMS scores was seen in the sixth decade (). The mean age of the studied population was 56.8 ± 12.6 years.
Table I. Severity of climacteric symptoms according to the AMS Questionnaire in the studied population (n = 146).
Table II. Prevalence of pathological AMS (≥37) and changes in mean serum levels of total testosterone (tT), SHBG and calculated free testosterone (fT) in relation to age.
Mean AMS subscores were 14.9 ± 6.0 points for the somatovegetative, 9.3 ± 4.4 points for the psychological and 12.6 ± 4.6 points for the sexual subscore. According to the before mentioned threshold values, 47.3% of the studied men had a pathological somatovegetative subscore, 32.2% had a pathological psychological subscore and 62.3% had a pathological sexual subscore.
Hormone levels
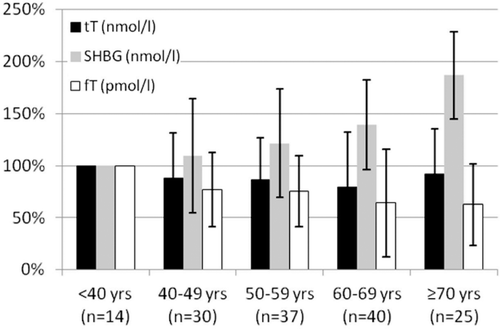
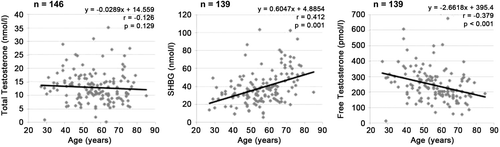
The mean (median) tT value was 12.9 ± 5.7 (11.6) nmol/l, the mean (median) SHBG was 38.9 ± 18.7 (34.7) nmol/l, and mean (median) fT was 245.6 ± 105.7 (229.0) pmol/l. A deficit in tT and fT was shown in 25 (17.1%) men and 34 (24.5%) men, respectively. With respect to age, tT showed no clear statistically significant trend with increasing age (p = 0.129, Spearman Rho test) ( and ). On the other hand, age-related increase of mean SHBG from 30.4 nmol/l in patients <40 years to 56.8 nmol/l in patients ≥70 years was statistically significant (p < 0.001, spearman Rho test) ( and ). Concomitantly, mean fT decreased significantly with age from 335.4 to 209.6 pmol/l (p < 0.001, spearman Rho test).
Correlation of total AMS score and hormone levels
Using univariate analysis, pathological AMS scores (AMS ≥ 37) significantly correlated with a tT <8 nmol/l (p = 0.041, Fisher’s exact test). However, in the age- and BMI-adjusted multivariate analysis, there was only a trend towards an association between a tT deficiency and a pathological AMS score (p = 0.061). At a threshold of 37 points the AMS Questionnaire rendered an overall diagnostic sensitivity of 76.0% and specificity of 61.6% for the prediction of pathologic total testosterone levels (tT < 8 nmol/l). Post-hoc analysis revealed a markedly better diagnostic accuracy of the AMS Questionnaire in men >65 years. Thus, for these men specificity reached 73.5% with a comparable sensitivity of 75%. In contrast, for younger men (≤65 years) the questionnaire had a specificity of only 56.3%, without a markedly superior sensitivity at 76.4%.
Univariate analysis revealed an insignificant trend towards a correlation between a pathological total AMS score with fT deficiency (p = 0.077, Fisher’s exact test). At a threshold of ≥37 the AMS Questionnaire rendered an overall diagnostic sensitivity of 58.8% and a specificity of 58.9% to predict pathologic free testosterone levels (fT < 180 pmol/l). Post-hoc analysis revealed a better sensitivity of 78.9% in younger men (≤65 years), but its specificity did not exceed 56.8% in the prediction of pathologic fT levels.
Correlation of AMS subscores and hormone levels
Total testosterone
The median tT levels in men with pathological somatovegetative subscores were lower than in men without relevant somatovegetative symptoms (10.62 vs. 12.77 nmol/l; p = 0.011, Mann–Whitney test). Also, the median tT levels in men with pathological psychological subscores were lower than in men without relevant psychological symptoms (10.40 vs. 12.30 nmol/l; p = 0.002, Mann–Whitney test). The BMI- and age-adjusted multiple regression analysis also revealed a statistically significant independent correlation of either subscore with a deficit in tT (p = 0.042 and p = 0.01).
A pathological sexual subscore was also related to a lower median tT (11.14 vs. 12.70 nmol/l). However, this correlation was not statistically significant (p = 0.07; Mann–Whitney test), and there was no association between a tT deficit and a pathological sexual subscore applying multiple regression analysis, either (p = 0.17).
As the somatovegetative and the psychological subscores significantly correlated with tT, we calculated the diagnostic accuracy of a virtual questionnaire, which would only contain these two subscores, at a threshold of ≥26 points. Excluding the sexual subscore items, the questionnaire’s sensitivity and specificity would reach 66.0% and 65.6%, respectively.
Free testosterone
The median fT levels in men with pathological somatovegetative subscores were lower than in men without relevant somatovegetative symptoms (222.08 vs. 242.90 nmol/l; p = 0.041, Mann–Whitney test). Also, the median fT levels in men with a pathological psychological subscore were lower than in men without relevant psychological symptoms (213.40 vs. 232.49 nmol/l; p = 0.025, Mann–Whitney test). However, in contrast to the results for tT, BMI- and age-adjusted multiple regression analysis revealed no statistically significant correlation of these two subscores with a pathologic fT (p = 0.88 and p = 0.076, respectively).
On the other hand, men with pathologic sexual subscores had a markedly lower fT than symptom-free men (204.73 vs. 263.72 nmol/l; p = 0.006, Mann–Whitney test). Multiple regression analysis also confirmed a statistically significant correlation between a pathological sexual subscore and a deficit in fT (p = 0.039).
Discussion
The main goal of this study was to evaluate, if climacteric symptoms correlate with low circulating androgens and if the AMS Questionnaire could therefore be used as a prediction tool for serologic hypogonadism. Originally, the questionnaire was developed for the assessment of aging-related symptoms that occur during the “male climacteric”. Because symptoms of aging often have multiple causes, they do not necessarily find their expression in hormone values. Age and BMI as the best-known confounders were considered in our multiple correlation analysis.
Our results suggest that urological patients have a high perception of sexual complaints while experiencing rather moderate somatovegetative and psychological problems. In relation to the population sample derived from Heinemann’s original description [Citation15], which includes men at a comparable age, our studied outpatient population had much higher percentages of moderate or severe psychological (50.0 vs. 14.6%), somatovegetative (58.2 vs. 27.4%) or sexual impairments (84.2 vs. 27.8%). This might be explained by a selection effect due to our specific outpatient population. This, however, rises the question in how far assessment tools derived from representative population-based samples are applicable to a clinical setting. The prevalence of subnormal tT and fT in our study group was in agreement with previous findings [Citation29,Citation30]. While tT showed no significant decrease with patients’ age, the average decreases of −2.66 pmol/l per year for fT and −0.60 nmol/l per year for SHBG were significant.
The results of the present study suggest that the AMS Questionnaire does not serve as an appropriate tool for the prediction of a hormonal deficit. The diagnostic accuracy of the questionnaire to predict a lowered tT (<8 nmol/l) with a sensitivity of 76% and a specificity of 61.6% does not seem to be sufficient. This is in agreement with previous findings [Citation26,Citation27,Citation31]. Interestingly, the post-hoc analysis of the subgroup of men >65 years revealed a better performance of the AMS Questionnaire in predicting lowered tT with a sensitivity of 75% and a specificity of 73.5%. Nevertheless, the predictive value for lowered fT in this subgroup was again low (sensitivity 33.3%, specificity 66.6%). Therefore, we cannot recommend the general use of the AMS Questionnaire for the prediction of low fT/tT, independent of the subject’s age.
Previous studies have also compared the AMS subscores instead of the total AMS score with prevailing hormone values [Citation23–26,Citation32]. Their results showed a poor correlation of the somatovegetative and the psychological subscore with fT and tT. For the sexual subscore, at least a part of the studies revealed a good correlation with lowered androgen levels [Citation26,Citation32]. This encountered us to investigate the value of the AMS Questionnaire in total and for each of its three subsections separately. In contrast to previous studies [Citation23,Citation24,Citation26,Citation32], our correlation analysis revealed a moderate but statistically significant correlation between the somatovegetative and the psychological subscore with a pathologically lowered tT. The reasons for this discrepancy to earlier trials remain mainly unclear. The previous studies seem not to be underpowered regarding to their sample size (134–348 subjects). Possibly, there is an interference caused by different thresholds for each of the pathological subscores. In our study, we determined ≥15 points for somatovegetative and ≥11 points for psychological /sexual subscore as being pathologic which correlated to a pathological total AMS score of ≥37. In contrast, Heinemann et al. defined a moderately or severely pathological somatovegetative, psychological or sexual subscore with threshold values of ≥13 points, ≥9 points and ≥8 points [Citation15].
The superiority of the somatovegetative and the psychological subscore over the sexual in predicting a tT deficit prompted us to test the predictive value of the AMS Questionnaire, when the sexual subscore is ruled out post-hoc. Unfortunately, this did not lead to an improvement in the diagnostic accuracy in comparison to the full AMS questionnaire (sensitivity: 76% → 66%, specificity: 61.6% → 65.6%).
Correlations of the three subscores with fT are in contrast to those results for tT. Hence, while correlational analyses failed to detect a relation between the somatovegetative/psychological subscores with fT, the sexual subscore was a valuable predictor for lowered fT. This is in agreement with previous findings [Citation26,Citation32]. Basar et al. [Citation32] revealed a correlation with the sexual subscore for fT and to a smaller extent for tT. The study of Morley et al. [Citation26] reproduced this relation only for fT, similar to our findings. Therefore, we assume that sexual complaints are associated to a greater extent to low fT levels than tT levels. This is supported by the studies of Ahn et al. [Citation33] and Yavuz et al. [Citation34] which revealed a significant association of low fT levels with erectile dysfunction, as was determined by the International Index of Erectile Function (IIEF) Questionnaire.
In conclusion, our findings suggest that in ambulatory men climacteric symptoms assessed by the AMS Questionnaire do not sufficiently predict the androgen status. This might be explained by the fact, that the questionnaire was designed to measure the clinical changes associated with aging and that aging has a multifactorial genesis with serologic hypogonadism being only one component of the whole entity “male climacteric”.
Declaration of Interest: The authors report no conflicts of interest.
References
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002;87:589–598.
- Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Morley PM, Stauber PM, Vellas B, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metab Clin Exp 1997;46:410–413.
- Lapauw B, Goemaere S, Zmierczak H, Van Pottelbergh I, Mahmoud A, Taes Y, De Bacquer D, et al. The decline of serum testosterone levels in community-dwelling men over 70 years of age: descriptive data and predictors of longitudinal changes. Eur J Endocrinol 2008;159:459–468.
- Wu AH, Whittemore AS, Kolonel LN, John EM, Gallagher RP, West DW, Hankin J, et al. Serum androgens and sex hormone-binding globulins in relation to lifestyle factors in older African-American, white, and Asian men in the United States and Canada. Cancer Epidemiol Biomarkers Prev 1995;4:735–741.
- Blümel JE, Chedraui P, Gili SA, Navarro A, Valenzuela K, Vallejo S. Is the Androgen Deficiency of Aging Men (ADAM) questionnaire useful for the screening of partial androgenic deficiency of aging men? Maturitas 2009;63:365–368.
- Keenan DM, Takahashi PY, Liu PY, Roebuck PD, Nehra AX, Iranmanesh A, Veldhuis JD. An ensemble model of the male gonadal axis: illustrative application in aging men. Endocrinology 2006;147:2817–2828.
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010;95:2536–2559.
- Wang C, Nieschlag E, Swerdloff RS, Behre H, Hellstrom WJ, Gooren LJ, Kaufman JM, et al. ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male 2009;12:5–12.
- Spetz Holm AC, Fredrikson MG, Hammar ML. Symptoms of testosterone deficiency in early middle aged men. Aging Male 2012;15:78–84.
- Yassin AA, Saad F. Improvement of sexual function in men with late-onset hypogonadism treated with testosterone only. J Sex Med 2007;4:497–501.
- Auyeung TW, Lee JS, Kwok T, Leung J, Ohlsson C, Vandenput L, Leung PC, Woo J. Testosterone but not estradiol level is positively related to muscle strength and physical performance independent of muscle mass: a cross-sectional study in 1489 older men. Eur J Endocrinol 2011;164:811–817.
- LeBlanc ES, Wang PY, Lee CG, Barrett-Connor E, Cauley JA, Hoffman AR, Laughlin GA, et al. Higher testosterone levels are associated with less loss of lean body mass in older men. J Clin Endocrinol Metab 2011;96:3855–3863.
- Janowsky JS. Thinking with your gonads: testosterone and cognition. Trends Cogn Sci (Regul Ed) 2006;10:77–82.
- Seidman SN, Araujo AB, Roose SP, Devanand DP, Xie S, Cooper TB, McKinlay JB. Low testosterone levels in elderly men with dysthymic disorder. Am J Psychiatry 2002;159:456–459.
- Heinemann LAJ, Zimmermann T, Vermeulen A, Thiel C, Hummel W. A new ‘Aging Males’ Symptoms’ Rating Scale. The Aging Male 1999;2:105–114.
- Rosen RC, Araujo AB, Connor MK, Elstad EA, McGraw SA, Guay AT, Morgentaler A, Miner MM. Assessing symptoms of hypogonadism by self-administered questionnaire: qualitative findings in patients and controls. Aging Male 2009;12:77–85.
- Daig I, Heinemann LA, Kim S, Leungwattanakij S, Badia X, Myon E, Moore C, et al. The Aging Males’ Symptoms (AMS) Scale: review of its methodological characteristics. Health Qual Life Outcomes 2003;1:77.
- Valenti G, Gontero P, Saccò M, Fontana F, Strollo F, Castellucci A, Heinemann LA. Harmonized Italian version of the Aging Males’ Symptoms Scale. Aging Male 2005;8:180–183.
- Rabah DM, Altaweel W, Arafa MA. Clinical assessment and validation of an Arabic Aging Male Symptoms questionnaire in patients with androgen deficiency. Aging Male 2011;14:33–36.
- Akinyemi A, Bamiwuye O, Inathaniel T, Ijadunola K, Fatusi A. The Nigerian Aging Males’ Symptoms Scale. Experience in elderly males. Aging Male 2008;11:89–93.
- Leungwattanakij S, Lersithichai P, Ratana-Olarn K. The Aging Males’ Symptoms Rating Scale: cultural and linguistic validation into Thai. J Med Assoc Thai 2003;86:1106–1115.
- Conway K, Heinemann LA, Giroudet C, Johannes EJ, Myon E, Taieb C, Raynaud JP. Harmonized French version of the Aging Males’ Symptoms Scale. Aging Male 2003;6:106–109.
- Miwa Y, Kaneda T, Yokoyama O. Correlation between the Aging Males’ Symptoms Scale and sex steroids, gonadotropins, dehydroepiandrosterone sulfate, and growth hormone levels in ambulatory men. J Sex Med 2006;3:723–726.
- Kang J 2nd, Ham BK, Oh MM, Kim JJ, Moon du G. Correlation between serum total testosterone and the AMS and IIEF questionnaires in patients with erectile dysfunction with testosterone deficiency syndrome. Korean J Urol 2011;52:416–420.
- T’Sjoen G, Goemaere S, De Meyere M, Kaufman JM. Perception of males’ aging symptoms, health and well-being in elderly community-dwelling men is not related to circulating androgen levels. Psychoneuroendocrinology 2004;29:201–214.
- Morley JE, Perry HM 3rd, Kevorkian RT, Patrick P. Comparison of screening questionnaires for the diagnosis of hypogonadism. Maturitas 2006;53:424–429.
- Clapauch R, Braga DJ, Marinheiro LP, Buksman S, Schrank Y. Risk of late-onset hypogonadism (andropause) in Brazilian men over 50 years of age with osteoporosis: usefulness of screening questionnaires. Arq Bras Endocrinol Metabol 2008;52:1439–1447.
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–3672.
- St Sauver JL, Jacobson DJ, McGree ME, Girman CJ, Klee GG, Lieber MM, Jacobsen SJ. Associations between longitudinal changes in serum estrogen, testosterone, and bioavailable testosterone and changes in benign urologic outcomes. Am J Epidemiol 2011;173:787–796.
- Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol 1998;147:750–754.
- Beutel ME, Wiltink J, Hauck EW, Auch D, Behre HM, Brähler E, Weidner W; Hypogonadism Investigator Group. Correlations between hormones, physical, and affective parameters in aging urologic outpatients. Eur Urol 2005;47:749–755.
- Basar MM, Aydin G, Mert HC, Keles I, Caglayan O, Orkun S, Batislam E. Relationship between serum sex steroids and Aging Male Symptoms score and International Index of Erectile Function. Urology 2005;66:597–601.
- Ahn HS, Park CM, Lee SW. The clinical relevance of sex hormone levels and sexual activity in the ageing male. BJU Int 2002;89:526–530.
- Yavuz BB, Ozkayar N, Halil M, Cankurtaran M, Ulger Z, Tezcan E, Gurlek A, Ariogul S. Free testosterone levels and implications on clinical outcomes in elderly men. Aging Clin Exp Res 2008;20:201–206.