Abstract
The term “late-onset hypogonadism (LOH)” is recommended to express the symptoms in middle-aged males with decreased testosterone. Although androgen replacement therapy (ART) might be an effective way to manage LOH, the risk of testosterone supplementation in elderly men is still concerned. On the other hand, to avoid adverse effects of ART, Kampo medicine (traditional Chinese–Japanese medicine) is often a first choice to treat LOH in Japan. However, their pharmacological studies are few. In this study, castrated mice was used as an LOH animal model for examining the pharmacological effects of a Kampo formula, saikokaryukotsuboreito (shortly SKRBT) on serum testosterone levels and seminal vesicles weights. Furthermore, an attempt to elucidate its pharmacological mechanism, inhibition of SKRBT and its components against aromatase were also examined with the enzyme-based assay. As a result, SKRBT improved significantly both the decline of serum testosterone levels and decrease of seminal vesicles weight of castrated mice at a dose of 125 mg/kg with a non dose-dependent manner. SKRBT and two components Scutellariae radix and Rhei rhizoma exhibited inhibitory activities with the IC50 values of 145, 29.2 and 29.7 µg/ml, respectively. These results suggested that the aromatase inhibitory activity of SKRBT may contribute, to a different extent, to the improvement of serum testosterone levels.
Introduction
The term “andropause” and/or “male climacteric” are used to describe the emotional and physical changes such as depression, fatigue, low sexual activity and loss of energy occurred in middle-aged males. Unlike women, due to men not having a clear-cut signpost such as the cessation of menstruation to mark this transition, these changes and the care in the middle-aged males had not attracted adequate attention. However, more studies have shown that “andropause” is closely associated with low testosterone levels, and its decline actually happens in male with aging [Citation1–4]. Decreased testosterone could put men at risk for health problems like heart disease, obesity, weak bone, depression and low sexual function [Citation5–7].
As taking account of the characteristics of the “andropause”, namely, gonadal function in male is affected in a slow progressive way as part of the normal aging process, the International Society for the Study of the Aging Male (ISSAM) recommended to use “late-onset hypogonadism (LOH)” to express the symptoms in middle-aged males with decreased testosterone, and further released the diagnosis, treatment and monitoring guidelines of LOH for the first time in 2002 [Citation8]. Androgen replacement therapy (ART) might be an effective way to manage LOH on the basis of the guidelines; however, the risk of testosterone supplementation in elderly men is still a concern. For instance, ART could be risky for prostate cancer, worsening symptoms of benign prostatic hypertrophy and so forth [Citation9].
On the other hand, traditional herbal medicine such as Kampo medicine (traditional Chinese–Japanese medicine) is often a first choice to treat LOH in order to avoid adverse effects of ART in China, Japan and other regions or nations of Southeast Asia; in some cases traditional medicines are expected to be of benefit to libido.
Kampo medicine is characteristic of making from multiple herb medicines called crude drugs according to a distinguished medicine theory from Western medicines or modern medicines, and the combination of these component herbs is called a “Kampo formula”. Since 1976, the products of Kampo formulae have been approved by the Japanese Government for reimbursement under the national health insurance program, current approximately 89% of Japanese doctors in a poll [Citation10] are said to prescribe Kampo medicines to patients in their daily practice. In particular, Kampo medicine is preferred to be used in the treatment of climacteric disorders of both female and male.
Recently, saikokaryukotsuboreito (SKRBT, Chai-Hu-Chia-Lung-Ku-Mu-Li-Tang in Chinese), a Kampo formula, improved reportedly LOH-related symptoms in clinical trials [Citation11–13]. SKRBT is composed of Bupleuri radix, Pinelliae tuber, Poria cocos, Cinnamomi cortex, Scutellariae radix, Zizyphi fructus, Ginseng radix, Fossilia ossis mastodi, Ostreae testa, Rhei rhizoma and Zingiberis rhizoma (), which is used in the treatment of climacteric disorders, neurotic importance, hypertension, etc. [Citation14,Citation15]. However, few studies on pharmacological actions against LOH are reported till now.
Table 1. Composition (daily dose) of Kampo formula SKRBT.
In this study, castrated mouse was used as an LOH animal model to examine the effects of SKRBT on serum testosterone levels and weight of seminal vesicles. Moreover, an attempt to elucidate its pharmacological mechanism, inhibitory activity against aromatase was also examined with the enzyme-based assay because aromatase is a limited enzyme to convert testosterone and other C19 steroids into estrogens resulting in decreased testosterone levels in males [Citation16].
Materials and methods
Animals
Male ddY mice (five weeks old, 16–21 g on arrival) were purchased from SLC Inc. (Shizuoka, Japan) for establishing LOH-like models. They were reared in an air-conditioned animal house facility (room temperature 23 ± 2 °C; reversed 12 h light/dark cycle; relative humidity 55 ± 10%) at Kampo Research Laboratories in Kracie Pharma Ltd. The mice were housed in sterilized polypropylene cages (four mice/cage) and provided laboratory pellet chow (CE-2, Clea Japan Inc., Tokyo, Japan) and water ad libitum. Before experimental procedures, they were acclimated to the room for one week. This experiment (approval no. 100042) was reviewed and approved by Experimental Animal Care Committee of Kracie Pharma Ltd. (Takaoka, Japan).
Chemicals and building LOH animal model
The dried extract powder of SKRBT (lot no. 8042503) was used in this study, which was manufactured in GMP pharmaceutical factory of Kracie Pharma Ltd. The extracted powder was suspended in the 0.5% sodium carboxymethyl cellulose (CMC–Na) just before use and administered to experimental animals on the basis of daily clinical dose (3900 mg/d). Testosterone propionate (T.P., lot no. 03K0695, Sigma–Aldrich, St. Louis, MO) was dissolved in sesame oil (lot no. 067K0069, Sigma-Aldrich, St. Louis, MO) and administrated (s.c.) at a dose of 5 mg/kg/d in the volume of 1 ml/kg.
Sixty mice were treated with bilateral gonadectomy by the use of a 0.5 cm incision in the median of scrotum prior to the start of this study and randomly assigned to five groups after 3 d of the treatment on the basis of their body weights, and another intact 12 mice were subjected to normal control. Among them, apart from normal and control groups treated with 0.5% CMC–Na solution and positive control with T.P., SKRBT were given at a dose of 125, 250 and 500 mg/kg by oral administration to each group for 10 d.
In addition to the above experiments, the effects of SKRBT on the serum testosterone levels and seminal vesicles weight of normal mice were also examined with the same administration scheme.
At the end of the experiments, blood from each animal was collected by decapitation in diethyl ether anesthesia and allowed to clot at room temperature for 1 h. Serum samples were prepared by centrifugation at 10 000 rpm for 10 min and stored at −20 °C until assay. Total serum testosterone levels were measured using Rodent Testosterone ELISA test kit (lot no.281570, R&D Systems Inc., Minneapolis, MN), which were purchased from Hirano Pure Chemical Co. Ltd. (Aichi-ken, Japan). All the samples were run at the same time to avoid inter-assay variation. The seminal vesicles were collected and weighed for organ wet weight after blood sampling. In addition, body weight was recorded every 1 d.
Aromatase inhibition assay was conducted with CYP19/MFC high throughput inhibitor screening kit (lot. no. 47764, BD Biosciences, San Jose, CA) depending on an attached protocol. Aromatase inhibitor, anastrozole (lot no. 2593407, LKT Laboratories Inc., Saint Paul, MN) was used as a positive control. A 50% inhibitory concentration (IC50) was determined using three or four wells in each test.
The experimental data are expressed as mean ± SEM. Statistical significance was determined by Student’s t-test when two groups were compared. The recovery effects of body weight were analyzed by two-factor repeated measure ANOVA with statistical package (Statcel 3, OMS Publishing Inc., Saitama, Japan). Values of p less than 0.05 were accepted as statistically significant.
Results
Effect of SKRBT on body weights
Castration led to dramatic decreases in body weights, as presented in , the body weight on the first day of the experiments were 34.3 ± 1.8, 31.0 ± 0.8, 31.8 ± 1.0 and 31.7 ± 0.8, 31.0 ± 0.8, 31.3 ± 1.4 g in the normal, control, positive control group and SKRBT-treatment groups, respectively. T.P. enhanced significantly the body weight recovery of castrated mice from day 1 since administration in comparison with the control group, and their body weight increase exceeded the increase of normal group from day 6. Among the groups treated with SKRBT (125, 250 and 500 mg/kg), body weight recovery was just observed to be enhanced significantly with the period of consecutive administration at a dose of 125 mg/kg (p < 0.05, ANOVA analysis).
Table 2. Change of body weights during experiment.
Changes in serum testosterone levels and seminal vesicles weight by the treatment of SKRBT
Castration resulted in a dramatic decline of serum testosterone and reduction of seminal vesicles weight (), and by treatment with T.P. serum testosterone levels () and weight of the seminal vesicles () were significantly increased in castrated mice compared with those of the normal and control groups.
Figure 1. Effects of SKRBT on serum testosterone levels and seminal vesicle weights. Mean ± SEM, n = 12, ###p < 0.001 versus normal, ***p < 0.001, *p < 0.05 versus control (Student’s t-test).
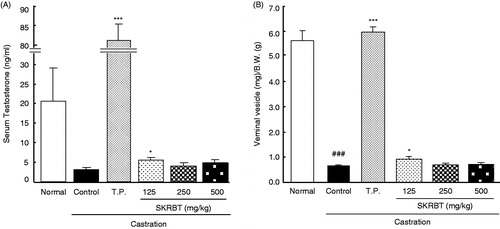
In SKRBT-treated mice, the levels of serum testosterone and weight of the seminal vesicles were increased with a non-dose-dependent manner. Among them, the effects of SKRBT at a dose of 125 mg/kg were significant ().
Inhibitory activities of SKRBT and each component against aromatase
Aromatase is a cytochrome P450 enzyme complex located in the endoplasmic reticulum. In males, it reversibly converts testosterone to estradiol resulting in decreased testosterone and elevated estrogen levels [Citation17]. Aromatase inhibitors (e.g. anastrazole) increase reportedly endogenous testosterone levels in elder men [Citation18–20]. Thus, focusing on aromatase inhibition could be an approach to elucidate the pharmacological actions of SKRBT on serum testosterone levels, and the inhibitory activities of SKRBT and its components against aromatase were examined with the enzyme-based assay.
As presented in , SKRBT extract exhibited aromatase inhibition with an IC50 value of 145 µg/ml. Furthermore, inhibitory activities of each component herbs against aromatase were also examined, and both Scutellariae radix and Rhei rhizome exhibited more than 50% inhibition at a concentration of 100 µg/ml (IC50 values: 29.2 and 29.7 µg/ml, respectively) among them.
Table 3. Inhibitory activity of SKRBT and each component against aromatase.
Discussion
With aging in male, decline in their testosterone levels has been confirmed under a number of cross-sectional and longitudinal studies in the world [Citation1–4]. Decreased testosterone levels are associated with increased risks of heart disease, osteoporosis metabolic syndrome, type 2 diabetes mellitus and depression [Citation5–7]. In 2002, the term LOH was recommended to describe a variety of physical and psychological symptoms and/or disorders resulted from testosterone levels in aging male, and their diagnosis, treatment and monitoring guidelines were released by ISSAM for the first time [Citation8]. Under the guidelines, ART might be an effective way to manage LOH; however, the risks of testosterone supplementation are still concerned [Citation9].
In Japan, clinical doctors usually prefer to use Kampo medicine to treat LOH in their practice than ART because Kampo medicine is considered to be effective and having low side effects. Recently, SKRBT, a Kampo formula was reported that improved LOH-related symptoms in clinical trials [Citation11–13].
In this study, castrated mice was used as an LOH animal model for examining the pharmacological effects of SKRBT on serum testosterone levels and seminal vesicles weights. As a result, SKRBT improved significantly both the decline of serum testosterone levels and decrease of seminal vesicles weight of castrated mice at a dose of 125 mg/kg. Meanwhile, the body weights were recovered significantly with the period of consecutive administration of SKRBT at a dose of 125 mg/kg. Hence, the improvement of serum testosterone levels could contribute to an enhancing effect of SKRBT on body weight during the experiment. However, these effects of SKRBT were not in a dose-dependent manner, suggesting that an optimal dosage of SKRBT may exist. In fact, a similar phenomenon, that is bell-shaped dose–response curves, was observed due to the effect of SKRBT on the monoamine-related substance of mouse brain [Citation21].
Generally, androgens (dehydroepiandrosterone: DHEA, androstenedione and testosterone) are secreted largely from sex organ testes and a little portion from adrenal cortex. In this study, the mice testes were removed out, as shown in . Though castration led to a dramatic decline of serum testosterone levels, the decline did not reach to zero level in the control group. It suggested that a compensatory secretion mechanism of androgens should be activated in adrenals, namely the origin of serum testosterone of castrated mice is almost considered to be from the secretion of adrenal cortex. Testosterone from adrenal cortex is primarily synthesized from cholesterol as well as in Leydig cells in the testes, and the rate-limiting step involves their biosynthetic pathway. Among them, aromatase is responsible for the aromatization of androgens into estrogens in the last step, resulting in decreased testosterone and elevated estrogen levels in males [Citation16]. A number of studies have demonstrated that aromatase inhibitors such as letrozole and anastrozole increase testosterone production and normalize serum testosterone in older men with hypogonadism [Citation18–20], suggesting that aromatase plays an important role in regulating testosterone biosynthesis and/or secretion in male, and further an approach targeting at aromatase inhibition could help elucidate the mechanism of how SKRBT improved serum testosterone levels. Moreover, Hayes et al. [Citation22] reported that aromatase inhibition facilitates the production of androgens by reducing estrogenic production because estradiol is a crucial mediator of hormonal feedback at the pituitary and hypothalamus.
Previously, we demonstrated that anastrazole enhances serum testosterone levels and seminal vesicle weights of castrated mice in a dose-dependent manner [Citation23]. Thus, the inhibitory activities of SKRBT and its components against aromatase were examined with the enzyme-based assay. As a result, SKRBT extract, and the components Scutellariae radix and Rhei rhizome exhibited aromatase inhibition with the enzyme-based assay, although the activities were not stronger than that of anastrozole (IC50 value: 0.92 ng/ml). These results suggested that the aromatase inhibitory activity of SKRBT may contribute, to a different extent, to the improvement of serum testosterone levels. With respect to that, active constituent of Scutellariae radix and Rhei rhizome are now under investigation and will be reported elsewhere.
On the other hand, SKRBT did not alter serum testosterone levels and seminal vesicle weights of intact mice (data not shown), suggesting that there is no androgen-like action of SKRBT per se. Meanwhile, this finding excites interest in examining the difference in aromatase activity and/or aromatase expression between intact and castrated mice and the details remain to be analyzed further.
Finally, it should be noted that a problem, “a different composition of formula with the same name”, is present in Kampo medicines. As presented in , SKRBT consisting of 11 kinds of herb medicines was used in this study; however, the formula without the herb medicine Rhei rhizome is also used in Japan with the same formula name “saikokaryukotsuboreito”. Owing to the composition of a Kampo formula that could be freely adjusted, according to clinical diagnoses by ancient physician, and/or altered out of the habit in prescribing medicine in different countries, the problem is not fully resolved now.
In the light of this point, thus, we could speculate upon the reasons why the improvement of serum testosterone was not observed in the clinical study by Tsujimura et al. [Citation13], where SKRBT without Rhei rhizome was used. Therefore, as a clinician, it is important to use contemporaneous research findings as the basis for clinical decisions, particularly in applying complementary and alternative medicine including the Kampo medicine to practice. Therefore, building evidence-based medicine in Kampo medicine is advocated actively in Japan [Citation24].
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Notice of Correction
This paper published online on 22 January 2013 contained an error in Figure 1. In Figure 1A, the unit label of the y-axis and the bar representing T.P. were incorrect. The unit labels of the y-axis were changed, the SD of each bar has been corrected and the symbol of significance on the control bar has been removed. Figure 1A has been replaced with the corrected graph for this version.
References
- Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab 1991;73:1016–25
- Gray A, Berlin JA, McKinlay JB, Longcope C. An examination of research design effects on the association of testosterone and male aging: results of a meta-analysis. J Clin Epidemiol 1991;44:671–84
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002;87:589–98
- Iwamoto T, Yanase T, Horie H, et al. Late-onset hypogonadism (LOH) and androgens: validity of the measurement of free testosterone levels in the diagnostic criteria in Japan. Int J Urol 2009;16:168–74
- Cattabiani C, Basaria S, Ceda GP, et al. Relationship between testosterone deficiency and cardiovascular risk and mortality in adult men. J Endocrinol Invest 2012;35:104–20
- Spark RF. Testosterone, diabetes mellitus, and the metabolic syndrome. Curr Urol Rep 2007;8:467–71
- Amore M, Innamorati M, Costi S, et al. Partial androgen deficiency, depression, and testosterone supplementation in aging men. Int J Endocrinol 2012;2012:280724 doi: 10.1155/2012/280724
- Morales A, Lunenfeld B. Investigation, treatment and monitoring of late-onset hypogonadism in males. Official recommendations of ISSAM. Aging Male 2002;5:74–86
- Nigro N, Christ-Crain M. Testosterone treatment in the aging male: myth or reality? Swiss Med Wkly 2012;142:w13539. . doi: 10.44414/smw.2012.13539
- Japan Kampo Medicines Manufactures Association. Actual use survey of Kampo formulae 2011 (in Japanese). Available from: http://www.nikkankyo.org/topix/news.html [last accessed 3 July 2012]
- Tsujimura A, Takada S, Matsuoka Y, et al. Clinical trial of treatment with saikokaryukotsuboreito for eugonadal patients with late-inset hypogonadism-related symptoms. Aging Male 2008;11:95–9
- Sugimoto K, Shigehara K, Izumi K, et al. Effect of combination of Saiko-ka-ryukotsu-borei-to with androgen replacement therapy for LOH syndrome. Jpn J Impot Res 2009;24:349–53
- Tsujimura A, Miyagawa Y, Okuda H, et al. Change in cytokine levels after administration of saikokaryuukotsuboreito or testosterone in patients with symptoms of late-onset hypogonadism. Aging Male 2011;14:76–81
- The Ministry of Health and Welfare. The guidebook of non-prescription Kampo formulae (in Japanese) (Edited by The Ministry of Health and Welfare). Tokyo: Yakugyo-jihosha Press; 1975
- Hsu HY, Hsu CS. Commonly used Chinese herb formulas with illustrations. Long Beach (CA): Ohai Press (Oriental Healing Arts Institute); 1980
- Alan C, Margaret H. Mammalian aromatases. Reproduction 2001;121:685–95
- Karagiannis A, Harsoulis F. Gonadal dysfunction in systemic diseases. Eur J Endocrinol 2005;152:501–13
- Leder BZ, Rohrer JL, Rubin SD, et al. Effects of aromatase inhibition in elderly men with low or borderline-low serum testosterone levels. J Clin Endocrinol Metab 2004;89:1174–80
- de Boer H, Verschoor L, Ruinemans-Koerts J, Jansen M. Letrozole normalizes serum testosterone in severely obese men with hypogonadotropic hypogonadism. Diabetes Obes Metab 2005;7:211–15
- Burnett-Bowie SA, Roupenian KC, Dere ME, et al. Effects of aromatase inhibition in hypogonadal older men: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf) 2009;70:116–23
- Ito T, Murai S, Saito H, et al. Effect of Saiko-ka-ryukotsu-borei-to on the monoamine-related substances in several regions of mouse brain. Nihon Toyo Igaku Zasshi (Jpn J Orient Med) 1994;45:97–106
- Hayes FJ, Seminara SB, Decruz S, et al. Aromatase inhibition in the human male reveals a hypothalamic site of estrogen feedback. J Clin Endocrinol Metab 2000;85:3027–35
- Norimoto H, Yamaguchi H, Morimoto Y. Effects of anastrazole on serum testosterone and seminal vesicles weight in male mice with testosterone deficiency. The 8th European congress on Menopause; 2009 May 16–20; London, UK: Scientific Programme; 2009;49pp
- Terasawa K. Evidence-based reconstruction of Kampo medicine: Part-III – how should Kampo be evaluated? Evid-Based Complement Alternat Med 2004;1:219–22