Abstract
Objective: Low testosterone levels may be a signal of poor health. This study aimed to investigate the effects of age and abnormal metabolism on sex hormones in Chinese male.
Methods: Three hundred and thirty-seven elder men were enrolled into this single-center, cross-sectional study, and their sex hormone levels and metabolic parameters were assessed.
Results: Serum follicle-stimulating hormone (FSH), luteinizing hormone (LH) and sex-hormone-binding globulin (SHBG) concentrations increased with age, while free testosterone index (FTI), testosterone secretion index (TSI), estradiol (E2)/SHBG and progestin (PROG) decreased. Abnormal metabolisms were related to androgen indices (TT, FT, BT, FTI, TSI, T/E2), SHBG and E2/SHBG even after adjusting by age and macrovascular disease. Obesity and overweight, hyperglycemia and dyslipidemia were the most important abnormal metabolism that related to decreased androgen indices. Including SHBG in the stepwise regression increased the explanation effect of TT and BT by 32.7% and 28.5%, respectively, and all metabolic indices were excluded. Abnormal metabolism indies (BMI and PBG) were correlated to the decrease in SHBG levels, while age and LH was positively correlated to SHBG levels.
Conclusions: Age and abnormal metabolism were independently important factors associated with the sex hormone levels in elderly Chinese men, which were all mediated by SHBG.
Introduction
Testosterone is primarily produced and secreted by the Leydig cells in the testicles, and its production is regulated by a negative feedback loop that includes luteinizing hormone (LH) and LH-releasing hormone (LHRH); these hormones form the hypothalamic-pituitary-gonadal (HPG) axis [Citation1]. The enzyme, 5-α-reductase, metabolizes testosterone to its biologically active androgen form, dihydrotestosterone, and the enzyme aromatase converts it to estradiol. The majority of testosterone (80%) is bound to sex-hormone-binding globulin (SHBG) [Citation1], a plasma glycoprotein produced by hepatocytes and secreted into the blood. The fraction of TT bound to SHBG in serum is proportional to the SHBG level.
Endogenous hormones play an important role in many diseases [Citation2]. Testosterone is involved in regulating several physiological processes including sexual function, mood, muscle mass, secondary sex characteristics, liver function, lipids, bone formation, erythropoiesis and immune function, and thus a decline in testosterone levels has many effects [Citation3]. In contrast to women, whose estrogen levels fall precipitously at menopause, men of all ethnicities have a more gradual fall in testosterone levels. Up to 30% of men over 60 years of age have testosterone levels below the normal range for young men [Citation3,Citation4].
Testosterone levels are related closely with some abnormal metabolism, such as hyperglycemia and insulin resistance, some researches thought that lower testosterone may be one of those causes [Citation5–12]. While recently, the European Male Aging Study (EMAS) [Citation13] suggested that low testosterone may be a marker of poor health that leads to non-specific symptoms. Because the prevalence of abnormal metabolism increases with age, we hypothesized that age-related changes in metabolic conditions might contribute to the lower androgens and other hormones in elder men. In addition, until now, little was known about the correlation between the endogenous sex hormone levels and abnormal metabolism in elderly Chinese men. Thus, we conducted this study to determine the relationships between aging, abnormal metabolism and sex hormone levels.
Methods
Study population
This study was designed as a single-center, cross-sectional study enrolling men over 60 years of age. We screened 1920 men, age 60–91 years, who had routine physical examinations in the Chinese People’s Liberation Army General Hospital from May 2011 to July 2011. Potential subjects were from all 16 districts and 2 counties of Beijing. After excluding those who had malignancy or diseases that were treated using testosterone or corticosteroids, 508 elderly men (26.5%) who had their endogenous sex hormone levels examined remained as potential subjects. Among these potential subjects, the following patients were excluded: 90 who did not have biochemistry examinations, 79 who had kidney dysfunction (Cr > 113umol/l), 1 who was diagnosed with hyperparathyroidism and 1 who had moderate anemia. However, none were excluded based on a diagnosis of liver dysfunction (alanine transaminase (ALT) or aspartate transaminase (AST) above three times the normal level). Thus, 337 subjects (69.4% of 558) were included in the analysis.
Clinical parameters
After fasting at least 8 h overnight, baseline parameters were obtained the next morning between 6:00 am and 8:00 am by trained physicians. Baseline parameters included height, weight, body mass index (BMI; in kg/m2), age, sex, medical history, medication use and relevant diseases. Blood pressure (BP) was measured twice at the dominant side brachial artery with the patient in the sitting position and after a 5 min rest using the semi-automated oscillo-metric method.
Sex hormone measurements
A fasting venous blood sample was obtained between 6:00 am and 8:00 am after an overnight fast. A postprandial venous blood sample was extracted 2 h after eating 100 g of carbohydrate food or drinking liquid containing 75 g glucose. The blood sample was immediately centrifuged for 15 min at 4 °C, and the platelet-free serum and plasma were stored at −20 °C. All samples were tested within 1 week. Total testosterone (TT), SHBG, estradiol (E2), follicle-stimulating hormone (FSH), LH, prolactin (PRL) and progestin (PROG) were measured using a Roche Cobas6000-C601 analyzer (Roche Diagnostics, Mannheim, Germany). FT and bioactive testosterone (BT) were calculated using Vermeulen’s formula, and T/E2 and E2/SHBG were also calculated. The FT index (FTI) was calculated as FTI = T/SHBG, and the testosterone secretion index (TSI) was calculated as TSI = T/LH. The lower limit of TT detection was 0.02 nmol/l and the interassay variation was 6.0%. The lower limit of E2 detection was 2 pmol/l (2 ml sample), and interassay variation was 8.3%. The lower limit of SHBG detection was 5 nmol/l, and interassay variation was 9.0%. The lower limit of LH, FSH, PRL and PROG detection were all 0.01, and interassay variation was 4.6%, 6.6%, 7.0% and 5.0%. All these sex hormone measurements were performed by the Department of Geriatric Biochemistry, PLA General Hospital.
Other laboratory measurements
Biochemical indices were tested using serum samples collected after fasting, except for the postprandial blood glucose (PBG) that required postprandial serum samples. PBG, fasting blood glucose (FBG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), serum total cholesterol (TC), uric acid (UA), blood urea nitrogen (BUN), creatinine (Cr), ALT and AST were measured by an automatic enzymatic procedure using a Roche Cobas6000-C601 analyzer (Roche Diagnostics). Diagnoses were defined according to the case records at the corresponding medical institutions, whether or not treatments or medicines were used. New diagnoses were made if abnormal results were obtained, as described below. Diabetes mellitus was defined as FBG > 6.9 mmol/l and/or PBG > 11.0 mmol/l and/or treatment with insulin or oral hypoglycemic agents. Impaired glucose tolerance (IGT) was defined as FBG ≤ 6.9 mmol/l and 7.8 mmol/l < PBG ≤11.0 mmol/l, and impaired fasting glucose (IFG) was defined as 6.1 mmol/l < FBG ≤ 6.9 mmol/l and PBG ≤ 7.8 mmol/l. IGT and IFG were defined as impaired glucose regulation (IGR). Hypertension was defined as systolic BP (SBP) >140 mmHg and/or diastolic BP (DBP) > 90 mmHg and/or the use of anti-hypertensive medication. The patient was considered positive for hyperlipidemia if any abnormal lipid test result was detected (reference range provided by the Geriatric Biochemistry Department of the Chinese People’s Liberation Army General Hospital: TC > 5.7 mmol/l, TG > 1.7 mmol/l, HDL < 1.6 mmol/l, LDL > 3.4 mmol/l) and/or if the patient was taking any lipid-lowering medications. Hyperuricemia was defined as uric acid > 420 μmol/l and/or if the corresponding medications were used. BMI > 24 kg/m2 was diagnosed as overweight, while BMI > 28 kg/m2 was diagnosed as obesity. Metabolic abnormal numbers were calculated according to the abovementioned standards for each subjects. The diagnose of metabolic syndrome was judged by the guidline of China Medical Association Diabetes School Grade Meeting (CDS, 2010), which includes more than 3 of below abnormals: BMI ≥ 25.0 kg/m2, dyslipidemia (including TG > 1.7 mmol/l and/or HDL <0.9 mmol/l), hyperglycemia and hypertension described as above.
Statistical analysis
The SPSS software 17.0 package (SPSS Inc, Chicago, IL) was used for both data management and analysis. Continuous variables were presented as the mean ± SD. The unpaired Student’s t-test was used to test for differences between two groups. Differences in means between three groups were tested using multiple factor linear analysis and adjusting for age, BMI and macrovascular disease. Age, BMI, BP (SBP, DBP), plasma glucose (FBG, PBG), plasma lipid (TC, LDL, HDL and TG), plasma uric acid, macrovascular disease, abnormal metabolism (MetS, the numbers of different abnormal metabolism) were included in different subregions of the stepwise regression analysis to analyze the effect of abnormal metabolism on sex hormones. Using SHBG as the independent variable, and hepatic and renal functions, LH, FSH, age and various metabolic variables as the dependent variables, a stepwise regression analysis was performed to determine the effective factors of SHBG. Variables were reported as percentages. The Chi-square (χ2) test was conducted for inter-group comparisons. For these analyses, p < 0.05 was considered to indicate the statistical significance. The 95% confidence interval (95% CI) was estimated to describe the magnitude of the associations.
Results
Sex hormones and age
The age range in this population was from 60 to 91 years, and the liver and kidney functions were within the normal range. In this population, TT levels were low (only one result was higher than 11 mmol/l and 92.3% were lower than 8 mmol/l), BT and FT levels were also low, while E2, SHBG, LH and FSH were high. PROG levels were in the normal range. Sex hormone levels and the subjects’ general condition are shown in .
Table 1. Characteristics of the study population and sex hormones.
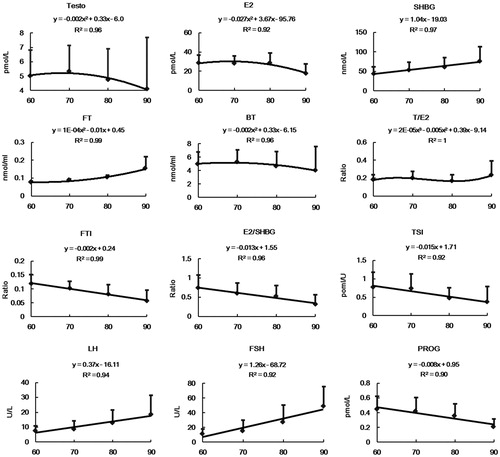
Changes in sex hormone levels and age are presented in . Sex hormones that had a linear relationship to the age included SHBG, FTI, E2/SHBG, TSI, FSH, LH and PROG; these sex hormones also showed a significant difference by age category (p < 0.01). Serum FSH, LH and SHBG showed a corresponding increase with increasing age, which was 2.12%, 3.94% and 2.4% per year, respectively. TSI, FTI, E2/SHBG and PROG decreased with age, and showed a decrease of 1.84%, 1.47%, 1.86% and 1.04% per year, respectively. TT and E2 showed a decreasing parabola curve with a statistically significant difference among the age groups (p < 0.01 and p < 0.05, respectively). No differences were found between age and FT, BT and T/E2 (p > 0.05).
Figure 1. Sex hormones (mean ± SD) and trendlines over different age categories. Mean ± SD are shown by curves for TT (pmol/l), E2 (pmol/l), SHBG (pmol/l), FT (nmol/ml), BT (nmol/ml), T/E2, FTI, E2/SHBG, TSI (pmol/IU), FSH (IU/l), LH (IU/l), PROG (pmol/l) and grouped by age among 337 men. p < 0.01 for all sex hormones that had a linear relation with age, including SHBG, FTI, E2/SHBG, TSI, FSH, LH and PROG.
Metabolic conditions in different age groups
Two age category models were used. In model 1, patients were divided into age categories as follows: 60–69 years, 70–79 years, 80–89 years, 90 years and above. In model 2, five patients of 90 years and above were excluded. Overall, the results for models 1 and 2 showed similar trends. Abnormal metabolic parameters (mean ± SD) by age category are listed in . The DBP level and the proportion of normal plasma glucose decreased significantly with age (p < 0.01, p < 0.05, separately). Although there was a significant difference for hypertension, PBG and plasma uric acid level among different age groups, there was no regular pattern with age. No difference was found in MetS, dyslipidemia, hyperuricemia and the abnormal metabolism numbers by age category. Besides, the morbidity of cardiovascular disease, cerebrovascular disease and macrovascular disease, all increased with age in this population (p < 0.01).
Table 2. Metabolic indices by age category.
Abnormal metabolism and sex hormones
Sex hormones by abnormal metabolic test result categories are presented in . Adjustments were made for age, age + BMI, age+ macrovascular disease and age + BMI +macrovascular disease sequentially by multivariate-adjusted linear trend.
Table 3. Sex hormone levels by abnormal metabolic test result category.
The results suggested that the quantity of abnormal metabolisms and MetS have a close relationship with the androgen indices TT, FT, BT, FTI, TSI and T/E2, and also with SHBG and E2/SHBG. There was a reduction in sex hormone levels compared to an increase in the quantity of abnormal metabolisms. The relationship of BMI, abnormal glucose levels with sex hormones was similar to that of MetS. These significances did not change after multiple covariance analysis adjusting. Androgens also decreased in hypertension patients in the univariate analysis, but the significant difference for TT, T/E2 and TSI disappeared after adjusting for BMI + age, and the difference observed for FT and BT were further reduced after adjusting by BMI + age +macrovascular disease. On the contrary, relationships of dyslipidemia and BT, FT were only discovered after adjusting for BMI + age + macrovascular disease. Besides, PROG was related to BMI and hypertension; E2, FTI, LH and FSH were not related to any of the impaired metabolic diseases.
Decreased SHBG in MetS, overweight, obesity and subjects with more than three abnormal metabolisms, together with an unexpected decline in hyperuricemia, were discovered and these four differences remained after adjusting for age + BMI + macrovascular disease. The difference of SHBG in normal, IGR and DM was 0.08 after adjusting for BMI + age + macrovascular disease, and the difference was significant (p < 0.05) when compared between subjects with and without DM (data not shown).
Stepwise regression was used then as described previously to evaluate the effect of abnormal metabolism on sex hormones. Equations for sex hormones are listed in , and no ideal equations of E2 and PROG were found (the corresponding R2 was 0.03 and 0.06, respectively). The results showed that FT, LH and FSH were only related to age, and independent of metabolic indices and MetS. However, TT, BT and T/E2 were not related to age, but were related to the metabolic indices of BMI, TC and PBG. The coefficients of the BMI, TC and PBG in TT and BT equations are almost the same as that of SHBG. So we added SHBG as an extra subregion in the TT and BT stepwise regression, and found that the explanation effect of the variation in TT and BT increased from 14% and 12% to 46.7% and 40.5%, respectively. After SHBG was included in the equation, all the metabolic indices were excluded, and TT levels significantly decreased with age. The special stepwise regression analysis of SHBG showed that SHBG was positively correlated to age, TC, HDL and LH, but negatively correlated to BMI and PBG. Age and BMI played an important role. The explanatory power of the equation was 43%.
Table 4. Sex hormone test results analyzed using stepwise regression.
Discussion
Research on the influence factors on endogenous androgens is impeded by the complex interrelations of common diseases, associated conditions and behavior [Citation14,Citation12]. So in this study, we first enrolled subjects without the chronic diseases, which were believed to influent sex hormones other than age and metabolic parameters as described previously. Then we measured serum levels of sex hormones (not limited to androgens), and assessed rates of metabolism and performed statistical analyses to determine if there was any correlation between various hormone levels and the metabolic rate, and to determine the relative importance of these factors. This is, to our knowledge, an innovative study to systematically assess the relationship between age, hormone levels and metabolic rate in this perspective.
This population of elderly Chinese men had an extremely low TT level; their TT level was less than one-third of the mean TT level observed in men in the European Male Aging Study [Citation15] (EMAS, TT 16.5 nmol/l) and an American study including 400 elderly men [Citation10] (TT 18.5 nmol/l). However, the average age of subjects in our study (72.92 years) was higher than that of the other two studies (59.7 years and 60 years, respectively). We reviewed other studies in China, and found similar TT levels reported in a study from Shanghai, in which subjects had a similar average age (75 years) and a similar BMI level to that in our study [Citation16]. The relationship between age and TT in men remains unclear. Several large cross-sectional studies have demonstrated annual decline in TT, BT and dehydroepiandrosterone sulfate levels [Citation17,Citation18]. Several studies showed, however, that TT remained stable with age in healthy participants [Citation10,Citation19]. In our study, analysis by age category also showed that TT, FT, E2 and T/E2 levels first increased and then decreased between the ages of 60 years and 90 years. These results suggest that age is not the only factor that causes a decrease in testosterone levels in elderly men.
It is reasonable to hypothesize that age-related changes in androgens and other hormones might contribute to the development of MetS in older Chinese men. In our study, multivariate-adjusted linear trend analysis confirmed that abnormal metabolic test results and MetS have a close relationship with the main sex hormones. Moreover, not only MetS but also the individual abnormal metabolism, such as higher BMI, DM and dyslipidemia, are closely connected with sex hormones independent of age and chronic diseases.
However, multiple covariate analysis is not enough. First, the metabolic indices that had the greatest effect on most of the sex hormones could not be determined. Second, there were two opposing metabolic results in this population: the relatively high prevalence of some abnormal metabolic parameters and the ideal controlled metabolic indices. These opposing metabolic results likely occurred because of relatively stable income and convenient medical service, which consequently led to earlier discovery, diagnosis and treatment. Thus, we analyzed the relationship of all the sex hormones with the metabolic indices by including age and metabolic indices as continuous terms in the stepwise regression, and really find something interesting.
First, previous research on the correlation of FT levels and metabolism were not clear. Many reports indicated that the FT levels were decreased in MetS patients compared to healthy people, while some researchers suggested that correlation of FT to metabolism required further confirmation. For example, Hajamor et al. [Citation20] found that the association between FT and MetS was not statistically significant after adjusting for potential confounders. Also, Brand et al. [Citation21] recently showed that the association of TT and FT with MetS tended to be less pronounced in older men. Our study confirmed that FT levels were not correlated to abnormal metabolism in elderly men after adjusting for potential confounders by stepwise regression compared to multiple covariate analysis, and reported precisely that age could explain 18% of FT fluctuation. Thus, improving metabolic conditions does not seem to have an effect on increasing FT levels. In addition, since FT was the unique androgen that only affected by age, it is well-founded to believe that the age-regulated only HPG axis (the increased LH and FSH levels with age) may attribute to the decreased FT, which reflected the actual levels available to tissues.
Second, like that of FT, TT levels were significantly negatively correlated to age, but beyond that, TT levels were also negatively correlated to blood glucose levels and BMI independent of age, and BMI plays the most important role. So age and abnormal metabolism were independently important factors associated with TT levels in elderly Chinese men.
Third, The most interesting thing was that we describe for the first time in this manuscript that the coefficient of BMI, TC and PBG in the TT and BT equations are almost the same as that for SHBG in the regression design, which suggests that the relationship of total sex hormones with abnormal metabolism maybe explained by SHBG. Including SHBG in the stepwise regression increased the explanation effect of TT and BT by 32.7% and 28.5% respectively, and all metabolic indices were excluded. This indicated that SHBG is the key point for the action of metabolic factors on total testosterone [Citation22].
Though it was well-known that SHBG has a close relation with metabolic syndrome [Citation21,Citation23], it is our study that put forward the regulative effect of SHBG between metabolic syndrome and sex hormones creatively, and confirms that lower SHBG connect closely with MetS and its components (especially BMI and PBG). The cause of the SHBG decrease in metabolic imbalance is unclear. SHBG is a plasma glycoprotein produced by hepatocytes and secreted into the blood. Hepatocyte nuclear factor-4 (HNF-4) recruits the transcription initiating complex to the human SHBG promoter. Some researchers believe that lipogenesis induced by glucose or free fatty acids (in overweight and obesity) inhibits hepatic SHBG expression by reducing cellular HNF-4 levels [Citation24].
Furthermore, we also find a different influence of age and metabolism on SHBG. The increase in SHBG with age might be a result of the age-associated decrease in GH and IGF-1 levels; GH treatment of adult men with isolated GH deficiency results in decreased SHBG and TT level [Citation25]. Some researchers postulate that the HPG axis may also play a partial role in the increase of SHBG with age. In our study, age-related HPG hormone, LH, was closely related to higher SHBG, which explains SHBG 13% more than the equation without it. Increasing SHBG with age may be a central regulatory mechanism that compensates for the declining trend of the sex hormones. Thus, SHBG should be treated more like a regulating protein, rather than a kind of sex hormones associated with androgens, as it had been treated in previous studies [Citation26].
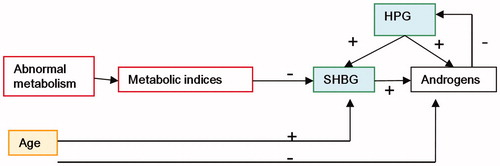
The relationship between age, abnormal metabolism and sex hormones is summarized in .
Figure 2. Interrelationship between age, abnormal metabolism and sex hormones. The effect of age and age-related chronic diseases includes the effect of abnormal metabolism on the androgen levels in elderly men. Serum FT and testosterone levels in elderly men significantly decreased with increasing age, and with the increase in incidence of MetS and chronic diseases, SHBG and TT levels decreased. However, age directly or indirectly promoted SHBG levels, which in turn compensated for the decrease in androgens in elderly men to some extend. + positive effect, − opposite effect. The yellow square means bidirectional effect on androgens; the red square means opposite effect on androgens; and the green square means positive effect on androgens.
To understand these findings, some issues need to be addressed. As mentioned before, findings from several logistics studies support a causal role for testosterone in MetS etiology, while some recent studies indicate that late onset hypogonadism is an expression of poor health. Our study confirmed this new perspective by announcing that abnormal metabolisms are associated with lower testosterone in elder men independently through systematically analysis. Though the cross-sectional estimates of associations are not true measures of longitudinal changes, we think it is reasonable to believe that the relationship between the metabolic syndrome and testosterone is bidirectional. Considering that there is not yet sufficient evidence to treat all ageing men with hypogonadism with T, increasing the T level in elder men by elevating SHBG level through ameliorating metabolic condition would be highly probable. We are planning to execute further studies to confirm these hormonal changes with age and metabolic disease, including an appropriately powered, placebo-controlled trial.
Conclusion
Obesity and overweight, hyperglycemia, dyslipidemia are the main metabolic factors affecting the gonadal hormone levels. Age and abnormal metabolism were independent important factors associated with sex hormone levels in elderly men, which was mediated by SHBG. MetS decreased SHBG levels, and age directly or indirectly increased SHBG levels.
Declaration of interest
The authors have declared that no conflict of interest exists.
This work was supported by the grants from the National Natural Science Foundation of China (NFSC, No. 81173625), the MiaoPu Fund of Chinese People’s Liberation Army General Hospital (12KMM33) and NanLou Innovation Fund of the Chinese PLA General Hospital (NQ201105).
References
- Burns-Cox N, Gingell C. The andropause: fact or fiction? Postgrad Med J 1997;73:553–6
- Bjornerem A, Straume B, Midtby M, et al. Endogenous sex hormones in relation to age, sex, lifestyle factors, and chronic diseases in a general population: the Tromso Study. J Clin Endocrinol Metab 2004;89:6039–47
- Araujo AB, Wittert GA. Endocrinology of the aging male. Best Pract Res Clin Endocrinol Metab 2011;25:303–19
- Grossmann M. Diagnosis and treatment of hypogonadism in older men: proceed with caution. Asian J Androl 2010;12:783–6
- Barrett-Connor E. Lower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitus. Ann Intern Med 1992;117:807–11
- Simon D, Preziosi P, Barrett-Connor E, et al. Interrelation between plasma testosterone and plasma insulin in healthy adult men: the Telecom Study. Diabetologia 1992;35:173–7
- Haffner SM, Valdez RA, Mykkanen L, et al. Decreased testosterone and dehydroepiandrosterone sulfate concentrations are associated with increased insulin and glucose concentrations in nondiabetic men. Metabolism 1994;43:599–603
- Laaksonen DE, Niskanen L, Punnonen K, et al. Sex hormones, inflammation and the metabolic syndrome: a population-based study. Eur J Endocrinol 2003;149:601–8
- Rucker D, Ezzat S, Diamandi A, et al. IGF-I and testosterone levels as predictors of bone mineral density in healthy, community-dwelling men. Clin Endocrinol (Oxf) 2004;60:491–9
- Muller M, Grobbee DE, den Tonkelaar I, et al. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab 2005;90:2618–23
- Kupelian V, Page ST, Araujo AB, et al. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab 2006;91:843–50
- Stanworth RD, Jones TH. Testosterone for the aging male: current evidence and recommended practice. Clin Interv Aging 2008;3:25–44
- Bhasin S, Pencina M, Jasuja GK, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab 2011;96:2430–9
- Ongphiphadhanakul B, Rajatanavin R, Chailurkit L, et al. Serum testosterone and its relation to bone mineral density and body composition in normal males. Clin Endocrinol (Oxf) 1995;43:727–33
- Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010;363:123–35
- Sun J, Pang XF, YX G. Correlation between serum sex hormone levels and parameters of bone metabolism and bone mineral density in aged men. J Intern Med Concepts Pract 2008;3:272–5
- Allen NE, Appleby PN, Davey GK, Key TJ. Lifestyle and nutritional determinants of bioavailable androgens and related hormones in British men. Cancer Causes Control 2002;13:353–63
- Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994;151:54–61
- Li JY, Li XY, Li M, et al. Decline of serum levels of free testosterone in aging healthy Chinese men. Aging Male 2005;8:203–6
- Hajamor S, Despres JP, Couillard C, et al. Relationship between sex hormone-binding globulin levels and features of the metabolic syndrome. Metabolism 2003;52:724–30
- Brand JS, van der Tweel I, Grobbee DE, et al. Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int J Epidemiol 2011;40:189–207
- Kang YG, Bae CY, Kim S, et al. Age-related change in serum concentrations of testosterone in middle-aged Korean men. Aging Male 2003;6:8–12
- Maggio M, Lauretani F, Ceda GP, et al. Association of hormonal dysregulation with metabolic syndrome in older women: data from the InCHIANTI study. Am J Physiol Endocrinol Metab 2007;292:E353–8
- Selva DM, Hogeveen KN, Innis SM, Hammond GL. Monosaccharide-induced lipogenesis regulates the human hepatic sex hormone-binding globulin gene. J Clin Invest 2007;117:3979–87
- Vermeulen A, Kaufman JM, Giagulli VA. Influence of some biological indexes on sex hormone-binding globulin and androgen levels in aging or obese males. J Clin Endocrinol Metab 1996;81:1821–6
- Muller M, den Tonkelaar I, Thijssen JH, et al. Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol 2003;149:583–9