Abstract
Background: Bone is a positive regulator of male fertility, which indicates a link between regulation of bone remodeling and reproduction or more specifically a link between calcium and androgens. This possibly suggests how calcium is linked to prostate cancer development through its link with the reproductive system. We studied serum calcium and sex steroid hormones in the Third National Health and Nutrition Examination Survey (NHANES III).
Methods: Serum calcium and sex steroid hormones were measured for 1262 men in NHANES III. We calculated multivariable-adjusted geometric means of serum concentrations of total and estimated free testosterone and estradiol, androstanediol glucuronide (AAG), and sex hormone binding globulin (SHBG) by categories of calcium (lowest 5% [<1.16 mmol/L], mid 90%, top 5% [≥1.30 mmol/L]).
Results: Levels of total and free testosterone, total estradiol or AAG did not differ across categories of serum calcium. Adjusted SHBG concentrations were 36.4 for the bottom 5%, 34.2 for the mid 90% and 38.9 nmol/L for the top 5% of serum calcium (Ptrend = 0.006), free estradiol levels were 0.88, 0.92 and 0.80 pg/ml (Ptrend = 0.048).
Conclusions: This link between calcium and sex steroid hormones, in particular the U-shaped pattern with SHBG, may, in part, explain why observational studies have found a link between serum calcium and risk of prostate cancer.
Introduction
Following positive findings for an association of intake of dairy products and calcium supplements with risk of prostate cancer [Citation1,Citation2], especially advanced disease, the link between serum levels of calcium and prostate cancer has been investigated lately [Citation3–8]. In the National Health and Nutrition Examination Survey I (NHANES I), comparing men in the top with men in the bottom tertile of serum calcium, the relative hazard was 2.68 (95% CI: 1.02–6.99) for fatal prostate cancer and 1.31 (95% CI: 0.77–2.20) for incident prostate cancer for men in the highest tertile of serum calcium compared to the lowest [Citation4]. Moreover, a statistically significant correlation was found between serum calcium and free prostate-specific antigen in NHANES 2005–2006, which indicates a possible link between the calcium metabolism and prostate cancer etiology [Citation5].
The link between calcium metabolism and PCa has also been found in pre-clinical studies. It was, for instance, shown in an athymic mouse model that tumor growth was effected by changes in dietary intake of vitamin D and calcium [Citation9]. When trying to identify the 17alpha-hydroxylase/17,20 lyase inhibitor’s (VN/124-1) mechanism of action against androgen-dependent cancer models, it was shown that this drug affects intracellular calcium levels and as a consequence the release of calcium from the endoplasmic reticulum [Citation10]. This is again another illustration of a potential link between the calcium and androgen metabolism.
Recently it was also shown that bone is a positive regulator of male fertility, which suggests a link between regulation of bone remodeling, energy metabolism, and reproduction or thus a link between the calcium and androgen metabolism [Citation11]. Since androgens promote cell proliferation and inhibit prostate cell death it is plausible that calcium is related to the risk of prostate cancer development via its link with the reproductive system [Citation12–14]. Androgens have been shown to modulate calcium through direct regulation of the STIM1 gene by androgen receptor binding to the STIM1 promoter [Citation15]. In relation to cancer, it has also been thought that there is a link between estrogen and calcium sensing receptor genotypes and serum calcium [Citation16]. The estrogen receptor-alpha, vitamin D receptor, and the calcium-sensing receptor have been linked to carcinogenesis through their effects on calcium levels [Citation16,Citation17].
In a cross-sectional analysis, we investigated serum calcium concentration in relation to concentrations of total testosterone, total estradiol, sex hormone binding globulin (SHBG), androstanediol glucuronide (AAG), free testosterone and free estradiol in adult males in the NHANES III, a nationally representative sample of non-institutionalized Americans. We hypothesized that men with higher serum calcium would have higher testosterone levels, lower estradiol, and higher SHBG.
Methods
Study population
The National Center for Health Statistics (NCHS) conducted NHANES III between 1988 and 1994 [Citation18] and designed it as a multistage stratified, clustered probability sample of the US civilian non-institutionalized population who was at least 2 months old. All subjects participated in an interview conducted at home and an extensive physical examination, which included a blood sample performed at a mobile examination center [Citation18]. NHANES III was conducted in two phases (1988–1991 and 1991–1994) which both lead to independent unbiased national estimates of health and nutrition characteristics. Within each phase, subjects were randomly assigned to participate in either the morning or afternoon/evening examination session. Of the 2205 men, who participated in the morning session of Phase I (1988–1991), we selected all men aged 20+ years who had serum measurements for total testosterone, total estradiol, SHBG, AAG and calcium (n = 1262).
Hormone measurements
Stored serum samples were assayed for sex steroid hormones at the Children’s Hospital Boston, MA. Testosterone, estradiol and SHBG concentrations were measured with competitive electrochemiluminescence immunoassays on the 2010 Elecsys autoanalyzer (Roche Diagnostics, Indianapolis, IN) in 2005. AAG, an indicator of the conversion of testosterone to dihydrotestosterone, was measured with an enzyme immunoassay (Diagnostic Systems Laboratories, Webster, TX). Laboratory technicians were blinded to participant characteristics. The detection limits of the assays were 0.02 ng/ml, 5 pg/ml, 0.33 ng/ml and 3 nmol/l for testosterone, estradiol, AAG and SHBG, respectively. The coefficients of variation for quality control specimens were: testosterone 5.9% and 5.8% at 2.5 and 5.5 ng/ml, respectively; estradiol 2.5%, 6.5% and 6.7% at 39.4, 102.7 and 474.1 pg/ml, respectively; AAG 9.5% and 5.0% at 2.9 and 10.1 ng/ml, respectively and SHBG 5.3% and 5.9% at 5.3 and 16.6 nmol/l, respectively. Quality control samples with a mean estradiol concentration of 39.4 pg/nL were also performed, which is then range of the of typical male estradiol concentrations (interassay coefficient of variantion: 2.5%) [Citation19]. Free testosterone was estimated from total testosterone, SHBG and albumin and free estradiol was estimated from total estradiol, SHBG and albumin using mass action equations [Citation20,Citation21].
Exposure measurements
Information on age, race/ethnicity, cigarette smoking, alcohol consumption and physical activity was collected during the interview. Race and ethnicity were combined into four racial/ethnic groups: non-Hispanic white, non-Hispanic black, Mexican American and other. Participants were classified as never, former and current smokers (<20, 20–40, ≥40 cigarettes per day) based on the self-reported smoking habits. Frequency of alcohol consumption was measured by a food frequency questionnaire and categorized by times per week. Vigorous physical activity was defined by the following activities: jogging or running; swimming or aerobics (for men 40 years or older); biking, dancing, gardening and calisthenics (for men 65 years or older) and walking and lifting weights (for men 80 years and older). Participants were defined as being diabetic when they reported a diagnosis of diabetes or when they were using insulin or diabetic medication. Percent body fat was estimated from anthropometric and bioelectrical impedance data using the equations of Chumlea and colleagues [Citation22]. Serum calcium was measured using a Hitachi 737 Analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN) [Citation23,Citation24]. 25-Hydroxy vitamin D was measured using the Diasorin radioimmunoassay kit (Diasorin, Stillwater, MN) on frozen serum from 1994 to 1995. Coefficients of variations from quality control samples ranged from 13% to 19%. The radioimmunoassay kit was calibrated using high-performance liquid chromatographically purified vitamin D every six months. The protocols for the conduct of NHANES III were approved by the institutional review board of the NCHS, Centers for Disease Control and Prevention. Written informed consent was obtained from all the participants. The Institutional Review Boards at the Johns Hopkins Bloomberg School of Public Health and the NCHS, Centers for Disease Control and Prevention approved the assay of stored serum specimens for the Hormone Demonstration Program.
Statistical analysis
All analyses were conducted with Statistical Analysis Systems (SAS) release 9.2 (SAS Institute, Cary, NC) and SUDAAN 9.0 software (Research Triangle Park, NC) as implemented in SAS 9.2. Phase I morning sampling weights for NHANES III were used to account for sampling variability and to adjust for differential probability of selection of persons [Citation18]. First, we calculated the age-adjusted means or percentages of characteristics of the men by categories of serum calcium concentration by adjusting for the age distribution of the US population according to the 2000 Census. Since the calcium metabolism is tightly controlled, the following categories of calcium were chosen to capture extreme variation: the bottom 5%, mid 90% and top 5%. Next, we calculated crude and adjusted geometric mean concentrations of the sex steroid hormones and their 95% confidence intervals (CI) by categories of serum calcium using linear regression. Hormone concentrations were not normally distributed, so we transformed them using the natural logarithm. The multivariable model was adjusted for age (continuous), race/ethnicity and factors that have been associated with hormone concentrations in previous NHANES III analyses: percent body fat, vigorous physical activity (yes or no), serum levels of vitamin D, cigarette smoking (never, former, current), alcohol intake (<2, 2–3, 4–6 times a week, or daily or more), and history of diabetes. We also adjusted for serum levels of albumin to obtain an estimate of the free ionized calcium levels, which is the amount of metabolically active serum calcium [Citation25]. In a sensitivity analysis, we performed an additional adjustment for comorbidity as both sex steroid hormones and calcium may be associated with other comorbidities. The comorbidity was evaluated with a comorbidity coefficient similar to the Charlson Comorbidity Index, as used in other NHANES III-based analyses [Citation26]. Each of the comorbidities available in the dataset contributed one point to the composite index with additional points given for older age. In addition, we performed stratified analyses for age groups (20–39, 40–59, ≥60 years), racial/ethnic groups and serum levels of vitamin D (< / ≥ 24.2 ng/mL) to determine whether the results were consistent between younger and older men, between different racial/ethnic groups and between men with different vitamin D levels. Since older men, men of African descent, and men with lower vitamin D levels tend to have a higher risk of prostate cancer [Citation27,Citation28], it is possible that the association between calcium and sex-steroid hormones differs for these groups. Since vitamin D is regulated by calcium and has been shown to have antiproliferative effects on human prostate cancer cells [Citation5,Citation29], the above analyses were also performed for serum calcium by low and high serum levels of vitamin D [Citation30]. We performed a test for interaction for all the above stratified analyses by introducing an interaction term in the linear regression models and testing its coefficient by the Wald test.
Results
Baseline characteristics of the study population by categories of serum calcium are shown in . The distribution of age, race/ethnicity, % body fat and serum albumin generally did not differ statistically by categories of calcium. Those in the lowest 5% of serum calcium concentration had the largest proportion of current smokers (44.1%). Those in the highest 5% of serum calcium had the largest proportion of men who performed vigorous physical activity (18.1%). The mean level of vitamin D (26.3 ng/mL) was lowest for those in the bottom 5% of serum calcium. The mean level of albumin was slightly higher (43.7 g/L) in those in the top 5% of serum calcium. Finally, vitamin D concentration increased across and categories of serum calcium (p = 0.038).
Table 1. Age-adjusted (standardized to the 2000 US Census age distribution) weighted characteristics by quintiles of serum calcium, men, NHANES III 1988–1991.
When comparing the fully adjusted geometric means of the sex steroid hormones by categories of serum normalized calcium, levels of total and free testosterone, total estradiol or AAG did not differ across categories of serum calcium. However, there was an upside down U-shaped association between categories of serum calcium and free estradiol: 0.88 for the bottom 5% of serum calcium, 0.92 in the middle 90% and 0.80 pg/mL in the top 5% (). In addition, there was a U-shaped association for SHBG (p = 0.006) () across categories of serum calcium: 36.4 for the bottom 5% of serum calcium, 34.2 in the middle 90% and 38.9 nmol/mL for the top 5%. Additional adjustment for comorbidity did not alter the results dramatically (results not shown).
Table 2. Geometric mean (95% CI) of sex steroid hormone concentrations by extreme measures of calcium in a nationally representative sample of adult men in NHANES III.
Although age was not a statistically significant effect modifier, shows that the above patterns for mean SHBG and free estradiol by serum calcium levels were only observed in men aged < 40 (p-trend = 0.003 and 0.092, respectively).
Table 3. Age-stratified geometric mean (95% CI) of sex steroid hormone concentrations by extreme measures of calcium in a nationally representative sample of adult men in NHANES III.
When stratifying by race/ethnicity, effect modification was observed for SHBG, total and free estradiol (Pinteraction = 0.030, 0.005 and 0.003, respectively) (). Interestingly, the U-shaped pattern for SHBG was only observed for non-Hispanic white men, whereas an upside-down U-shaped pattern was observed among Mexican American men and a positive association was seen for non-Hispanic black men ().
Table 4. Race/ethnicity-stratified geometric mean (95% CI) of sex steroid hormone concentrations by by extreme measures of calcium in a nationally representative sample of adult men in NHANES III.
No statistically significant effect modification was observed by median 25-hydroxy vitamin D levels, but the above observed U-shaped association for SHBG was only apparent for men with vitamin D levels ≥24.2 ng/mL, whereas the association for total estradiol was only apparent among those with vitamin D levels <24.2 ng/mL. A sensitivity analysis including only non-Hispanic white men, did not alter these findings (results not shown). Serum calcium level modeled as a continuous measure was not statistically significantly associated with any of the hormones.
Discussion
This cross-sectional study investigated how serum levels of calcium are correlated with levels of sex steroid hormones. We found that circulating calcium was especially associated with circulating levels of SHBG and free estradiol, but not total and free T, total E2 or AAG. This association was, in particular, observed in men aged 20–40 years and non-Hispanic white men. When stratified by levels of vitamin D, the U-shaped pattern for SHBG was only observed among men with 25-hydroxyvitamin D levels ≥24.2 ng/mL, whereas the upside-down U-shaped pattern for free estradiol was only observed among men with 25-hydroxyvitamin D levels <24.2 ng/mL – however, the interaction was not statistically significant.
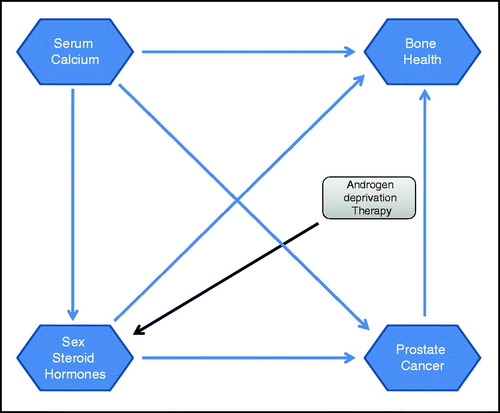
Several observations and pathways suggest a link between serum calcium levels and sex steroid hormones [Citation31,Citation32]: (1) both sex steroid hormones as well as serum calcium levels have been found predictive for the risk of prostate cancer development [Citation27,Citation33] and (2) sex steroid hormones and serum calcium levels have also been connected with bone health ().
Firstly, the link between sex steroid hormones and prostate cancer has been addressed in the context of androgen deprivation therapy and illustrated that the prostate is both an androgen-dependent and androgen-sensitive organ [Citation34]. Several epidemiological studies also assessed the link between serum levels of calcium and risk of prostate cancer [Citation3,Citation4,Citation6,Citation7]. Moreover, a study by Berry and colleagues [Citation15] showed that androgens modulate calcium through the direct regulation of the STIM1 gene by androgen receptor binding to the STIM1 promoter. However, currently no epidemiological study has examined whether an interaction in the association between circulating calcium levels and sex steroid hormone levels on the risk of prostate cancer incidence and progression may exist. Our data show an association between circulating levels of serum calcium and SHBG and free estradiol. Both sex steroid hormones have been found to be associated with severity and progression of prostate cancer [Citation35–37]. Using 18 prospective studies that included 3886 men with incident prostate cancer, the Endogenous Hormones and Prostate cancer Collaborative Group found an inverse association between serum concentration of SHBG prostate cancer risk (RR in the highest versus lowest fifth: 0.86; 95% CI: 0.75–0.98) [Citation38]. In the current study, the U-shaped association for SHBG was, in particular, observed for men aged 20–40 years and non-Hispanic white men, but there was also a statistically significant but different pattern in Mexican American men. This U-shaped pattern between levels of SHBG and serum calcium is also consistent with the U-shaped pattern for serum levels of calcium and risk of prostate cancer previously found [Citation8]. A modifying effect of race/ethnicity was also observed for the association between free estradiol and serum calcium. This is especially interesting since it has been previously shown that testosterone concentrations did not differ notably between black and white men in NHANES III [Citation19].
Another possible pathway between calcium and prostate cancer risk is via vitamin D, which is regulated by calcium and has been shown to have antiproliferative effects on human prostate cancer cells [Citation5,Citation29]. It has recently also been highlighted how calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2) is a key effector of the androgen receptor, regulating glycolytic flux by activating AMPK-PFK (5' AMP-activated protein kinase – phosphofructokinase) signaling, which in turn drives anabolism and thereby controls prostate cancer cell proliferation and tumor growth. CAMKK2 is thus regulated by the calcium/calmodulin complex and suggested to be an androgen receptor target in both androgen-dependent and castrate-resistant prostate cancer [Citation32]. Although there was no statistically significant interaction, the pattern for SHBG (i.e. low levels of SHBG with low and high calcium concentration and higher levels among men with calcium levels in the normal range) was only observed among men with 25-hydroxyvitamin D levels ≥24.2 ng/mL, whereas there was no association between circulating levels of SHBG and calcium in men with 25-hydroxyvitamin D levels <24.2 ng/mL. In contrast, the pattern for free estradiol (i.e. low free estradiol concentrations among men in the bottom and top categories of serum calcium) was only observed among men with 25-hydroxyvitamin D levels <24.2 ng/mL ().
Table 5. Vitamin D (median)-stratified geometric mean (95% CI) of sex steroid hormone concentrations by extreme measures of calcium in a nationally representative sample of adult men in NHANES III.
Secondly, sex steroids have been found to influence bone mass in healthy men. Bone mineral density has been positively correlated with estradiol levels, whereas an inverse correlation was found with SHBG, and no correlation was found with testosterone in men older than 65 years of age [Citation31]. It is also suggested that bone resorption is mainly regulated by estradiol, with a smaller but independent contribution of testosterone, whereas bone formation is regulated in equal proportions by the two hormones [Citation31]. Moreover, osteoporosis is a common side effect following androgen deprivation therapy for men with prostate cancer [Citation39]. It is observed that a rapid loss of bone-mineral density occurs within the first six to twelve months of endocrine treatment [Citation40–42]. A survival analysis based on the US SEER program, found that among men surviving at least 5 years after diagnosis, 19.4% of those who received androgen deprivation therapy had a fracture, as compared with 12.6% of those not receiving endocrine treatment (p < 0.001) [Citation43]. It was recently shown in the Osteoporotic Fractures in Men Study that adverse skeletal effects of low sex steroid levels (SHBG, free testosterone and estradiol) were more pronounced in men with low vitamin D levels [Citation44]. Overall, our results showed an inverse association between circulating levels of free estradiol and calcium, but when stratified by levels of vitamin D this association was only apparent among men with low vitamin D levels. Nevertheless, it is also important to point out that free estradiol was calculated based on levels of SHBG, total estradiol and albumin. Therefore, it is possible that the associations observed for free estradiol may follow from the patterns observed for SHBG.
This study has several strengths including its generalizability following the use of nationally representative data. Therefore, it was also possible in our analysis to perform a stratified analysis by race/ethnicity. We were able to adjust for many potential confounding factors and examine effect modification by age, race/ethnicity and vitamin D levels. A limitation of this study is that it relies on one single measurement so that it may be prone to measurement error and within-person variation. Moreover, our study used immunoassays instead of mass spectometry to evaluate serum levels of sex steroid hormones. Repeated measurements may strengthen the accuracy of the observed associations.
Conclusion
The present results suggest that there may be a positive association between sex steroid hormones and serum levels of calcium. This link between calcium and sex steroid hormones, in particular SHBG, may explain why observational studies have found a link between serum calcium and risk of prostate cancer. The observed U-shaped pattern for SHBG and serum calcium was consistent with the U-shaped pattern for serum calcium and prostate cancer risk found in other studies. A prospective study evaluating both serum levels of sex steroid hormones and calcium linked to risk and severity of prostate cancer or fractures could provide more insight into their roles related to prostate tumorigenesis and bone health.
Declaration of interest
This is the 22nd study from the Hormone Demonstration Program, which is supported by the Maryland Cigarette Restitution Fund at Johns Hopkins.
References
- Gao X, LaValley MP, Tucker KL. Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J Natl Cancer Inst 2005;97:1768–77
- Ma RW, Chapman K. A systematic review of the effect of diet in prostate cancer prevention and treatment. J Hum Nutr Diet 2009;22:187–99
- Halthur C, Johansson AL, Almquist M, et al. Serum calcium and the risk of prostate cancer. Cancer Causes Control 2009;20:1205–14
- Skinner HG, Schwartz GG. Serum calcium and incident and fatal prostate cancer in the National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev 2008;17:2302–5
- Skinner HG, Schwartz GG. The relation of serum parathyroid hormone and serum calcium to serum levels of prostate-specific antigen: a population-based study. Cancer Epidemiol Biomarkers Prev 2009;18:2869–73
- Skinner HG, Schwartz GG. A prospective study of total and ionized serum calcium and fatal prostate cancer. Cancer Epidemiol Biomarkers Prev 2009;18:575–8
- Van Hemelrijck M, Hermans R, Michaelsson K, et al. Serum calcium and incident and fatal prostate cancer in the Swedish AMORIS study. Cancer Causes Control 2012;23:1349--58
- Van Hemelrijck M, Hermans R, Michaelsson K, et al. Serum calcium and incident and fatal prostate cancer in the Swedish AMORIS study. Cancer Causes Control 2012;23:1349–58
- Ray R, Banks M, Abuzahra H, et al. Effect of dietary vitamin D and calcium on the growth of androgen-insensitive human prostate tumor in a murine model. Anticancer Res 2012;32:727–31
- Bruno RD, Gover TD, Burger AM, et al. 17Alpha-hydroxylase/17,20 lyase inhibitor VN/124-1 inhibits growth of androgen-independent prostate cancer cells via induction of the endoplasmic reticulum stress response. Mol Cancer Ther 2008;7:2828–36
- Oury F, Sumara G, Sumara O, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144:796–809
- Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Front Biosci 2006;11:1388–413
- Huggins C, Hodges CV. Studies on prostatic cancer – I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin 1972;22:232–40
- Richter E, Srivastava S, Dobi A. Androgen receptor and prostate cancer. Prostate Cancer Prostatic Dis 2007;10:114–8
- Berry PA, Birnie R, Droop AP, et al. The calcium sensor STIM1 is regulated by androgens in prostate stromal cells. Prostate 2011;71:1646–55
- Szendroi A, Speer G, Tabak A, et al. The role of vitamin D, estrogen, calcium sensing receptor genotypes and serum calcium in the pathogenesis of prostate cancer. Can J Urol 2011;18:5710–6
- Leclercq G. Calcium-induced activation of estrogen receptor alpha – new insight. Steroids 2012;77:924–7
- National Center for Health Statistics. Plan and operation of the third national health and nutrition examination survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat 1 1994:1–407
- Rohrmann S, Nelson WG, Rifai N, et al. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J Clin Endocrinol Metab 2007;92:2519–25
- Rinaldi S, Geay A, Dechaud H, et al. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev 2002;11:1065–71
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–72
- Chumlea WC, Guo SS, Kuczmarski RJ, et al. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord 2002;26:1596–609
- Sabanayagam C, Shankar A. Serum calcium levels and hypertension among U.S. adults. J Clin Hypertens (Greenwich) 2011;13:716–21
- Shiels MS, Rohrmann S, Menke A, et al. Association of cigarette smoking, alcohol consumption, and physical activity with sex steroid hormone levels in US men. Cancer Causes Control 2009;20:877–86
- Bringhurst F, Demay M, Krane S, Kronenberg H. Bone and mineral metabolism in health and disease. In: Fauci A, Longo D, Kasper D, et al., eds. Harrison's principles of internal medicine. Chap. 352. New York: McGraw-Hill; 2008
- Goldfarb-Rumyantzev AS, Rout P, Sandhu GS, et al. Association between social adaptability index and survival of patients with chronic kidney disease. Nephrol Dial Transplant 2010;25:3672–81
- Brandstedt J, Almquist M, Manjer J, Malm J. Vitamin D, PTH, and calcium and the risk of prostate cancer: a prospective nested case-control study. Cancer Causes Control 2012;23:1377–85
- Shui IM, Mucci LA, Kraft P, et al. Vitamin D-related genetic variation, plasma vitamin D, and risk of lethal prostate cancer: a prospective nested case-control study. J Natl Cancer Inst 2012;104:690–9
- Michaelsson K, Baron JA, Snellman G, et al. Plasma vitamin D and mortality in older men: a community-based prospective cohort study. Am J Clin Nutr 2010;92:841–8
- Blomberg Jensen M. Vitamin D metabolism, sex hormones and male reproductive function. Reproduction 2012;77:903–9
- Alexandre C. Androgens and bone metabolism. Joint Bone Spine 2005;72:202–6
- Massie CE, Lynch A, Ramos-Montoya A, et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J 2011;30:2719–33
- Roder MA, Christensen IJ, Berg KD, et al. Serum testosterone level as a predictor of biochemical failure after radical prostatectomy for localized prostate cancer. BJU Int 2012;109:520–4
- Goldenberg SL, Koupparis A, Robinson ME. Differing levels of testosterone and the prostate: a physiological interplay. Nat Rev Urol 2011;8:365–77
- Salonia A, Gallina A, Briganti A, et al. Circulating estradiol, but not testosterone, is a significant predictor of high-grade prostate cancer in patients undergoing radical prostatectomy. Cancer 2011;117:5029–38
- Hyde Z, Flicker L, McCaul KA, et al. Associations between testosterone levels and incident prostate, lung, and colorectal cancer. A population-based study. Cancer Epidemiol Biomarkers Prev 2012;21:1319–29
- Waldert M, Schatzl G, Swietek N, et al. Sex hormone-binding globulin is an independent predictor of biochemical recurrence after radical prostatectomy. J Urol 2012;188:792–7
- Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst 2008;100:170–83
- Thorstenson A, Bratt O, Akre O, et al. Incidence of fractures causing hospitalisation in prostate cancer patients: results from the population-based PCBaSe Sweden. Eur J Cancer 2012;48:1672–81
- Petrylak DP, Moul JW. Androgen abliation for prostate cancer: mechanisms and modalities. In: Kantoff PC, Carroll PR, D'Amico AV, eds. Prostate cancer: principles and practice. Philadelphia: Lippincott Williams & Wilkins; 2002:518–23
- Hakimian P, Blute M Jr, Kashanian J, et al. Metabolic and cardiovascular effects of androgen deprivation therapy. BJU Int 2008;102:1509–14
- Sprenkle PC, Fisch H. Pathologic effects of testosterone deprivation. Curr Opin Urol 2007;17:424–30
- Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 2005;352:154–64
- Barrett-Connor E, Laughlin G, Li H, et al. The association of concurrent vitamin D and sex hormone deficiency with bone loss and fracture risk in older men: the MrOS study. J Bone Miner Res 2012;27:2306–13
Notice of Correction
The version of this article published online ahead of print on 14 May 2013 contained an error in the author list. The author “Adrian Dobs” was incorrectly spelled as “Adrian Dobbs”. The error has been corrected for this version.